By Kelly Hewitt
For my capstone, I decided to share the project I helped work on for my research experience last fall. I am interested in working with people with neurological disorders, specifically rehabilitation post-stroke. I began talking with Dr. Michael Lewek, PT, PhD about the research experience elective in spring 2015, as research is something I enjoyed in my undergraduate career. In doing this, I became even more interested in the exploration of the biomechanics of hemiparetic gait and how it can be optimized. Specifically, we talked about current literature regarding center of mass (COM) as a focal point for rehabilitative interventions for individuals post-stroke. With that, I began my research experience in the fall and focused my CAT on COM-specific interventions post-stroke in our evidence-based practice II course. Our study was approved by the Institutional Review Board and consisted of data collection from eleven unimpaired control subjects and nine subjects with chronic stroke. After submission, my abstract of this study was selected for poster presentation at UNC’s Human Movement Science Research symposium this past February 2016, which helped me greatly in assessing the quality and “take-away” message of our study. From this, I gained helpful feedback to apply to the manuscript I have been working on for this capstone. With more feedback and potentially more variables analyzed, I plan to submit this manuscript for publication later this year. This capstone would not have been possible without the help and guidance of Mike Lewek, PT, PhD, and my fellow classmate, Korre Scott, SPT, who also helped conduct this research study. Below is an overview of the manuscript.
ABSTRACT
Introduction Walking recovery post-stroke often results in slow, asymmetric gait that requires significant energy consumption. Multiple impairments contribute to this, but perhaps one major cause of walking inefficiency is lack of paretic propulsion. This impairment results in a decreased ability to move the body’s center of mass (COM) forward during the latter half of stance phase. Proposed interventions to improve hemiparetic walking include error augmentation and error minimization. Using these opposing theories, we created a device that applies an anterior (aiding) or posterior (impeding) force to the COM that corresponds to paretic propulsion.
Objective This study aimed to determine if imposing anterior or posterior forces on the COM during paretic propulsion will alter the energy cost of walking and hip kinematics.
Methods Seven participants with stroke and eleven unimpaired controls underwent treadmill walking at their comfortable walking speed. Three randomly ordered conditions were tested for 4 (Stroke) or 5 (Control) minutes including: 1) comfortable walking (control), 2) walking with an anterior pull at the COM and 3) walking with a posterior pull at the COM. A novel device consisting of theratubing was utilized to apply an anterior or posterior force at the COM that coincided with paretic propulsion. Gas exchange (inspired VO2/expired VCO2) from a portable metabolic cart (Cosmed) was recorded for the fourth (stroke group) or fifth (control group) minute of walking and was normalized to body weight and speed (m/s) to yield cost of transport (COT) (ml O2/kg/m). Vicon motion capture systems recorded movement of pelvic and hip retroreflective markers to measure peak hip extension and hip flexion velocity. Hip kinematic variables and COT were each compared between conditions using a repeated-measures ANOVA with paired samples t-tests used as post-hoc tests, as necessary.
Results In both groups, there was a significant main effect for condition (p<0.001). Specifically, the energy cost significantly decreased for the anterior condition (p<.001) and increased for the posterior condition (p=.011) compared to the control condition measures for both groups. Peak hip extension was significantly lower in the anterior condition compared to the control (p<.001) and posterior (p=.042) conditions for both groups. Also in both groups, hip flexion velocity was significantly greater in the posterior condition compared to the control and anterior conditions (p=.001 and .002, respectively).
Conclusion These data support our hypotheses that an aiding force at the body’s COM can reduce energetics during gait and further, that hip kinematics are influenced by horizontal forces imposed on the COM throughout walking. Addressing COM mechanics may be a feasible approach for decreasing the metabolic cost of walking for individuals post-stroke.
BACKGROUND
Hemiparetic gait can require up to two times more metabolic energy than unimpaired walking, which drastically limits the distance that people can walk post-stroke.1–3 A substantial amount of this energy is attributed to the mechanical work required to redirect the body’s center of mass (COM) from step to step.2,4 The redirection of the COM requires adjustments in both vertical and anterior directions.5 Most of the available literature, however, has focused on control of the vertical displacement of the COM during walking6–8, despite the fact that an anteriorly directed propulsive force is a key determinant of walking function.9,10
Following stroke, individuals exhibit varying levels of unilateral muscular weakness, which contributes to disrupted forward progression during walking. Specifically, paretic ankle plantarflexor weakness results in decreased propulsion in the latter half of stance phase1,11 and has therefore been a target to optimize walking recovery.3 The higher energy costs associated with hemiparetic gait are not due to decreased efficiency of work production, but rather an increase in mechanical work done by the active muscles.1,2,5,12 The reduction in paretic ankle propulsion requires increased work by other muscles to maintain COM velocity in an anterior direction throughout the gait cycle.2,13,14 In agreement with this, recent analyses of walking post-stroke have shown pronounced mechanical asymmetries, with the non-paretic limb producing more positive mechanical power than the paretic limb.10,15 This mechanical imbalance induces phases of excessive acceleration and deceleration throughout the gait cycle, which interrupts the symmetric forward progression of the COM that is characteristic of healthy human walking.15
Traditionally, interventions targeting movement deficits resulting from stroke have focused on error minimization.16 However, recent evidence has found error augmentation to be effective at producing positive short-term outcomes, suggesting it is a viable approach for stroke rehabilitation efforts.17,18 In chronic stroke, exaggeration of error is thought to be beneficial because it provides a deviation substantial enough for the nervous system to detect and therefore, provide a correction.18,19 Since the forward progression of the COM is interrupted in hemiparetic walking, error augmentation, then, would provide a posteriorly-directed force at the pelvis during the gait cycle, whereas error minimization would entail providing an anteriorly-directed force. However, timing of force application should also be considered. Due to the influence of magnitude3,5,13 and timing13,20 of paretic propulsion on COM movement, it would be optimal to implement horizontal forces at the COM that correspond to paretic limb movements.
Based on previous literature of healthy subjects, an impeding force at the COM (error augmentation) will increase metabolic cost compared to an error- minimizing force.21 The purpose of this study was to determine the effect of the aforementioned competing motor theories (error augmentation and minimization) on energy expenditure and mechanical behaviors by imposing phase-dependent horizontal forces on the COM. Since propulsion deficits are limited only on the paretic side, we hypothesize that metabolic cost of walking can be affected by intervening in only half the gait cycle. To ensure proper timing of force application to the COM, we created a novel device that imposed either an anterior or posterior force to the pelvis (i.e., COM) that peaks during paretic propulsion. Since propulsion deficits are limited only on the paretic side, it is our hypothesis that imposing a horizontal force at the COM coinciding with paretic propulsion will have an effect on metabolic cost, despite being applied to only half the gait cycle. More specifically, we hypothesized that an anteriorly directed force coinciding with paretic propulsion (i.e., error minimization strategy) would reduce the metabolic cost of walking, whereas a posteriorly directed force (i.e., error augmentation strategy) would increase the metabolic cost of walking in individuals post-stroke. Additionally, we hypothesized that hip kinematics will be altered under error augmentation and minimization conditions.
METHODS
Participants
Nine individuals with chronic (>6 months) stroke (3 M / 6 F; age: 54 ± 11.4y; height: 67.6 ± 2.9in; weight: 195 ± 39lbs; self-selected walking speed: 0.64 ± 0.27m/s) and ten unimpaired individuals (4 M / 6 F; age 25.4 ± 2.8y; height 69.2 ± 3.9in; weight 155 ± 27lbs; self-selected over-ground walking speed 1.37 ± 0.15m/s) were recruited for this study. All subjects post-stroke presented with lower extremity hemiparesis (4 L / 5 R) resulting from an ischemic or hemorrhagic unilateral brain lesion. The average time since stroke was 102 ± 127months. Subjects were excluded from this study if they could not walk without therapist assistance, or self-reported a preexisting cardiovascular, metabolic, or musculoskeletal condition(s) that prohibited strenuous activity, a separate neurological condition that could affect walking ability, or a history of balance deficits or unexplained falls prior to stroke onset. Two individuals in the stroke group were not included for final analysis. One was unable to complete all testing conditions due to inadequate ankle stability and the second was unable to consistently walk on the separate belts of the treadmill without cross over. Participants were permitted to use their typical shoes, AFO, and assistive devices during testing. We used the lower extremity portion of the Fugl-Meyer Assessment to assess sensorimotor coordination for each subject in the stroke group (mean: 26 ± 4). All participants signed an informed consent form approved by the University of North Carolina at Chapel Hill before participating.
Within the control group, two participants had their left lower extremity tested, with all other individuals having their right side tested.
Data Collection
All testing occurred in a single session and consisted of three walking conditions performed on a dual-belt instrumented treadmill (Bertec Corp., Columbus, OH, USA). All participants wore an overhead safety harness that did not restrict lower extremity movement or provide unweighting. Prior to treadmill walking, all subjects performed 2 passes of overground walking at their self-selected gait speed across a 20-ft walkway (Zeno, Protokinetics, Havertown, PA, USA). The treadmill speed was then set for each subject to be ~80-100% of the subject’s overground gait speed. Participants were permitted to use the handrails as needed but no assistance was provided by the researchers. We recorded metabolic data and limb kinematic and kinetic variables as subjects walked on the treadmill.
In each of the three conditions we manipulated forces acting at the participants’ center of mass (COM) as they walked on the treadmill. The order of conditions for each participant was randomly selected using a random sequence generator. Conditions lasted five minutes each for unimpaired subjects and four minutes each for participants with chronic stroke. A five minute seated rest break was provided between each condition for all participants.
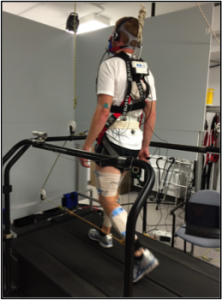
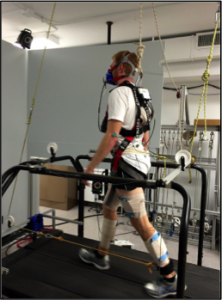
We developed a novel device consisting of a system of pulleys and elastic tubing (Theratube; Theraband, Akron, OH, USA) (see Figures 1 and 2). Resistance of the elastic tubing (black-, silver- or gold-colored tubing) was determined for each participant such that the maximum amount of force imposed would equate to ~10% of their individual body weight, a previously established energetic minimum.21 Two of the participants with stroke walked with a pull force of only ~5% of their body weight so as to enable the researchers to examine the effect of magnitude of pull force on energetics. Tubing was secured to the participants’ paretic (or tested) ankle using an adjustable hook and loop ankle strap and to the participants’ COM using a gait belt that was tightly adjusted around the pelvis.
The anterior condition involved a forward pulling force at the COM designed to decrease anterior COM velocity fluctuations. The set-up for the anterior condition involved elastic tubing that was attached at the participant’s COM looping around a pulley attached to a horizontal bar at the front of the treadmill and attached to the paretic ankle. With this set-up, as the paretic limb reaches peak hip extension during gait, the theratubing is stretched, causing an anterior-directed force to be imposed at the COM.
Figure 1: Anterior condition
Conversely, the posterior condition involved a backward pulling force at the COM designed to increase anterior COM velocity fluctuations. Here, the elastic tubing was attached to the front of the paretic ankle and looped through a series of four pulleys. The tubing stretched from the paretic (or tested) ankle to a pulley at the front of the treadmill, then through a pulley secured to the ceiling above the treadmill. From there, the tubing was looped through a pulley on the posterior aspect of the treadmill and finally was secured to the posterior aspect of the participant’s pelvis. With this set-up, at peak hip extension during gait, the tubing is stretched and a posteriorly-directed force is imposed at the COM.
During the anterior and posterior conditions, cues were provided by researchers for participants to maintain the appropriate force from the tubing. This involved telling participants to move towards the front or back of the treadmill based on live feedback of pull force. Cues were also provided to ensure each participant was walking in the center of the treadmill. During the control condition, participants walked without any imposed forces.
Figure 2: Posterior condition
Throughout each walking condition, kinematic and kinetic data were collected with an 8-camera motion capture system (Vicon, Denver, CO, USA) at a sampling frequency of 120Hz. Concurrently, we sampled the imposed force to the COM using a tension-compression load cell (MLP-100; Transducer Techniques) at 1200Hz. The three dimensional coordinates of 10-mm retroreflective markers were recorded as the participant walked on the treadmill. Details about marker setup have been described previously by Murray et al.22 All joint markers were kept in place for a static standing calibration to locate joint centers with respect to each segment coordinate system, then were removed prior to dynamic testing. For participants who wore an AFO during testing, shank clusters were secured over the orthosis.
Metabolic cost was measured for each condition using a portable metabolic cart (K4b2; Cosmed, Chicago, IL) which was calibrated prior to each session using a known concentration of gas (16%O2, 4%CO2). All subjects began testing with 5 minutes of quiet sitting to determine baseline energy cost at rest. Throughout each condition, oxygen consumption (VO2; mL×kg-1×min-1) and carbon dioxide production (VCO2) were collected on a breath-by-breath basis. The mask was removed during each rest break for participant comfort. Finally, pull force of the device on the COM was computed for each condition.
Data Management
Metabolic data from the fourth (stroke group) or fifth (control group) minute of walking were normalized to body weight and speed (m/s) to yield cost of transport (ml O2/kg/m). Average pull force of the device on the COM was recorded for each condition. Vicon Nexus analysis software was utilized to identify the locations of markers in the lab coordinate system. Visual3D software (C-Motion, Germantown, MD, USA) was utilized to create a 7-segment kinematic model to assess the 3-dimensional motion of the pelvis and bilateral lower extremities. All segment coordinate systems were defined with the positive x-axis to the right, positive y-axis facing anteriorly, and positive z-axis pointing superiorly. Six-Hz low pass filters were used for marker trajectory. Joint angles were calculated using Euler angles. Sagittal plane internal joint moments were calculated using an inverse dynamics approach and were normalized to each participant’s height and weight. All motion variables from the last minute of each condition were calculated using custom-written LabVIEW software (National Instruments, Austin, TX, USA). Kinetic and kinematic measures included peak hip extension and peak hip flexion velocity. Peak hip extension was measured as the peak angle between the pelvic marker and the respective thigh shank markers as the hip was moved into extension. Hip flexion velocity was defined by the change in angle between the respective thigh markers and the pelvic markers as the hip moved into flexion in the sagittal plane.
Data Analysis
Within-subject statistical analyses were performed using SPSS, version 23 (SPSS, Chicago, IL, USA). Cost of transport (COT) was compared between groups using a repeated-measure ANOVA (repeated for condition) with paired samples t-tests used as post-hoc tests, as necessary. Two-way repeated measures ANOVA (repeated for condition) was used to compare peak hip extension between groups. Peak hip flexion velocity was measured using a two-way repeated measures ANOVA with gait speed as a covariate. A significance level of α= 0.5 was used for all between condition and group comparisons.
RESULTS
Seven of the nine participants with chronic stroke were included in the analysis. Demographic data of these seven participants are described in Table 1. Additionally, eleven unimpaired control participants were included for analysis and are described in Table 2. The over-ground gait speed was 0.65m/s (SD: 0.3; range 0.31-1.1) and 1.37m/s (SD: 0.16; range 1.1-1.58), and the treadmill gait speed was 0.54m/s (SD:0.3; range 0.15-1) and 1.33m/s (SD: 0.12; range 1.1-1.5) for the stroke and control groups, respectively.
Table 1: Individual demographics for stroke group participants
| Subject | Age | Gender | Tested | Ht | Wt | Ground Speed (m/s) | TM Speed (m/s) | Post-stroke (months) | Fugl-Meyer |
| 1 | 39 | F | L | 64 | 151 | 0.54 | 0.45 | 20 | 26 |
| 2 | 37 | F | L | 67 | 236 | 0.62 | 0.55 | 28 | 25 |
| 3 | 49 | M | R | 68 | 150 | 1.13 | 1 | 33 | 31 |
| 4 | 60 | F | R | 64 | 163 | 0.31 | 0.15 | 46 | 17 |
| 5 | 69 | M | R | 73 | 260 | 0.45 | 0.4 | 23 | |
| 6 | 65 | F | R | 67 | 180 | 0.39 | 0.35 | 47 | 26 |
| 7 | 61 | M | R | 71 | 195 | 1.1 | 0.85 | 100 | 30 |
| Mean | 54.3 | 67.7 | 190.7 | 0.65 | 0.54 | 45.67 | 25.43 | ||
| SD | 12.7 | 3.35 | 42.8 | 0.33 | 0.3 | 28.58 | 4.65 |
Ht= height (inches), Wt= weight (lbs), TM speed= treadmill speed
Table 2:Individual demographics for participants in the control group
| Subject | Age | Gender | Tested | Ht | Wt | Overground speed (m/s) | TM Speed (m/s) |
| 1 | 25 | M | R | 68 | 160 | 1.35 | 1.35 |
| 2 | 25 | F | R | 65 | 130 | 1.4 | 1.4 |
| 3 | 23 | F | R | 67 | 138 | 1.58 | 1.4 |
| 4 | 24 | F | R | 66 | 160 | 1.54 | 1.5 |
| 5 | 31 | M | R | 75 | 185 | 1.4 | 1.4 |
| 6 | 25 | M | L | 70 | 162 | 1.11 | 1.1 |
| 7 | 30 | F | L | 65 | 123 | 1.2 | 1.2 |
| 8 | 24 | F | R | 69 | 147 | 1.34 | 1.3 |
| 9 | 24 | M | R | 76 | 210 | 1.43 | 1.4 |
| 10 | 23 | F | R | 71 | 130 | 1.54 | 1.4 |
| 11 | 25 | M | R | 72 | 165 | 1.17 | 1.17 |
| Mean | 25.36 | 69.45 | 155.45 | 1.37 | 1.33 | ||
| SD | 2.66 | 3.78 | 25.98 | 0.16 | 0.12 |
Ht= height (inches), Wt= weight (lbs), TM speed= treadmill speed
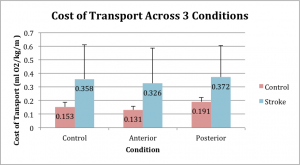
We observed a significant main effect for condition (p < .001, ηp2 = .553), such that the Posterior pull produced significantly greater COT than the Control condition (p=0.011) and the Anterior condition (p < .001). Likewise, the Control condition demonstrated a significantly greater COT than the anterior pull condition (p < .001). We also observed that the Stroke group had a significantly greater COT than the Control group (p = .018, ηp2 = .302), and observed no interaction effect (group x condition: p = .363, ηp2 = .061). (See Figure 3)
Figure 3
We observed a significant main effect for condition for the pulling force on the COM (p <.001, np2 = .928). There was no difference in the pulling force between the anterior and posterior pull conditions (p = 0.829), but both were significantly greater than the Control condition (p < .001). There was no significant difference in the pulling force between groups (p = .143, np2 = .147) or presence of an interaction effect (p = .387, np2 = .065).
We observed a significant main effect for condition for peak hip extension across both groups (p = .004, np2 = .379). Specifically, the anterior condition resulted in significantly less peak hip extension than both the control (p < .001) and posterior conditions (p = .042). There was no significant difference in peak hip extension between groups (p = .140, np2 = .149) and no interaction effect (group x condition : p =.935, np2 = .005). Peak hip flexion velocity was also analyzed using gait speed as a covariate to account for the different speeds of both groups. We observed a significant main effect for condition (p = .002, np2 = .635). For both groups, the posterior condition resulted in a significant increase in hip flexion velocity compared to the control (p = .001) and the anterior (p = .002) conditions. There was no significant difference in hip flexion velocity between groups (p =.663, np2 = .015) and no interaction effect (condition x group: p = .500, np2 = .052).
DISCUSSION
These data support our hypothesis that COT is reduced with an aiding force and increased with an impeding force applied to the COM. Across both the Control and Stroke groups, there was an increase in COT during the posterior (impeding) condition relative to the control condition. Conversely, there was a significant decrease in COT during the anterior (aiding) condition compared to the control condition across both groups. Timing and coordination of paretic propulsion has been previously proposed to be an important factor in optimizing hemiparetic gait.13 Our data suggests that rehabilitation strategies that provide assistance during paretic propulsion will result in reduced COT.
Altered energy cost associated with horizontal forces at the COM has been established in healthy adult populations.21 The fact that similar patterns occurred in this study on participants with chronic stroke indicates that a contributor to the excessive energy cost of hemiparetic gait is indeed, reduced forward progression of the COM. This knowledge is beneficial for rehabilitation strategies in that energy cost of walking post-stroke can potentially be improved with interventions that facilitate forward, horizontal progression of the COM.
As cost of transport (COT) is a whole body estimate, it is presumable that interventions designed to affect energetics can be most optimized by implementing a whole body approach. Conversely, some interventions have taken a joint-level approach to improve hemiparetic gait, focusing specifically on the influence of ankle plantar flexion weakness on walking mechanics.23,24 Paretic ankle exoskeletons, for instance, were found to increase ankle plantar flexion moments but had little effect on energetics.23 Similarly, functional electrical stimulation (FES) applied to paretic ankle musculature has shown immediate improvements in anterior ground reaction force values and targeted joint angles.24 Paretic ankle FES was also found to help reduce energy demand when used in combination with faster walking speeds.25 Nonetheless, the joint-level approach is a less direct means to manipulate overall body mechanics and energy cost. Additionally, the variety of muscle and joint responses following stroke26,27 may limit the generalizability of joint or muscle-specific approaches. Instead, a whole body approach that focuses on the mechanics of the COM may result in more meaningful gains because the patient is presented with the functional goal of smooth forward progression and is free to select the most appropriate neuromuscular response to achieve that goal.
The findings of this study not only suggest that the high energy cost of walking following stroke can be influenced by targeting the COM, but also that energy cost can be significantly reduced via implementing forces throughout only half of the gait cycle. Considering this, clinical interventions to reduce the energy cost of walking may be effective if they include manipulation of the COM at a time in the gait cycle when smooth forward progression is disrupted: the latter half of the paretic stance phase.
Additionally, these data reflect kinematic changes at the hip joint, which are known to influence propulsion.28 Specifically peak hip extension was decreased during the anterior and posterior conditions when compared to the control condition. Peak hip flexion velocity followed the same trend for both groups, increasing in the anterior condition and significantly increasing in the posterior condition compared to control condition values. This is important, because increased hip extension9,28 and decreased hip flexion output28 have been correlated to increased paretic propulsion. Therefore, our data suggest that the posterior condition, in which peak hip extension was reduced and hip flexion velocity was increased, further inhibited paretic propulsion thereby decreasing the forward progression of the COM. This assumption is supported by our metabolic data, which labeled the posterior condition as being the most energy-taxing. Clinical interventions, then, should aim to improve hip extension throughout hemiparetic gait to promote adequate propulsion and facilitate forward COM progression.
There have been numerous proposals about therapeutic interventions to teach patients with stroke how to walk with improved mechanics such as the hip extension adjustments explained above. Two major methods that have been debated include error augmentation versus error minimization. Though traditional rehabilitation has aimed to minimize errors, recent evidence suggests that error augmentation can improve spatiotemporal asymmetries present in hemiparetic gait.17,18,29 Augmenting errors related to forward progression (i.e., the posterior condition) reduced paretic propulsion, but resulted in significantly greater energy cost of walking.
Limitations
Participants included in this study represented a broad array of functional levels, however, as this study only analyzed seven chronic stroke survivors, there is limited applicability to the stroke population as a whole. Furthermore, two of the seven participants with chronic stroke had a different pull force percentage than the majority of the group, (5% of body weight instead of 10%) which may have decreased the consistency of our results. Another limitation of this study is that, overall, our Control group was much younger than our Stroke group and also had faster walking speeds. Finally, use of whole body estimates makes it difficult to pinpoint which specific muscles are contributors to energy cost changes. A more complete assessment of muscle activation influence on gait pattern and energy cost requires use of electromyography (EMG).27
Conclusions
Future studies should continue to investigate the role of the COM in the forward progression of hemiparetic walking. The decrease in energetic cost of walking associated with anterior COM motion found in this study suggest that hemiparetic gait can be improved with further focus on COM horizontal movement timed to paretic propulsion. Importantly, these favorable energetic effects can be elicited with interventions that only target the portion of the gait cycle during which the paretic limb is providing propulsion. More studies are needed to engineer additional whole body approaches that aim to facilitate adoption of a smooth, energetically efficient gait pattern.
REFERENCES
- Detrembleur C, Dierick F, Stoquart G, Chantraine F, Lejeune T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait Posture. 2003;18(2):47-55. doi:10.1016/S0966-6362(02)00193-5.
- Stoquart G, Detrembleur C, Lejeune TM. The reasons why stroke patients expend so much energy to walk slowly. Gait Posture. 2012;36(3):409-413. doi:10.1016/j.gaitpost.2012.03.019.
- Awad LN, Binder-Macleod SA, Pohlig RT, Reisman DS. Paretic Propulsion and Trailing Limb Angle Are Key Determinants of Long-Distance Walking Function After Stroke. Neurorehabil Neural Repair. 2015;29(6):499-508. doi:10.1177/1545968314554625.
- Kuo AD, Donelan JM, Ruina A. Energetic Consequences of Walking Like an Inverted Pendulum : Step-to-Step Transitions. Exerc Sport Sci Rev. 2005;33(2):88-97. doi:10.1097/00003677-200504000-00006.
- Farris D, Hampton A, Lewek M, Sawicki G. Revisiting the mechanics and energetics of walking in individuals with chronic hemiparesis following stroke: from individual limbs to lower limb joints. J Neuroeng Rehabil. 2015;12(1):24. doi:10.1186/s12984-015-0012-x.
- Gordon KE, Ferris DP, Kuo AD. Metabolic and Mechanical Energy Costs of Reducing Vertical Center of Mass Movement During Gait. Arch Phys Med Rehabil. 2009;90(1):136-144. doi:10.1016/j.apmr.2008.07.014.
- Massaad F, Lejeune TM, Detrembleur C. Reducing the Energy Cost of Hemiparetic Gait Using Center of Mass Feedback: A Pilot Study. Neurorehabil Neural Repair. 2010;24(4):338-347. doi:10.1177/1545968309349927.
- Massaad F, Lejeune TM, Detrembleur C. The up and down bobbing of human walking: a compromise between muscle work and efficiency. J Physiol. 2007;582(Pt 2):789-799. doi:10.1113/jphysiol.2007.127969.
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37(3):872-876. doi:10.1161/01.STR.0000204063.75779.8d.
- Balasubramanian CK, Bowden MG, Neptune RR, Kautz S a. Relationship Between Step Length Asymmetry and Walking Performance in Subjects With Chronic Hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43-49. doi:10.1016/j.apmr.2006.10.004.
- Peterson C, Hall A, Kautz S, RR N. Initiation and Power Generation By Individual. J Biomech. 2011;43(12):2348-2355. doi:10.1016/j.jbiomech.2010.04.027.PRE-SWING.
12. Stoquart GG, Detrembleur C, Nielens H, Lejeune TM. Efficiency of work production by spastic muscles. Gait Posture. 2005;22(4):331-337. doi:10.1016/j.gaitpost.2004.11.004.
- Soo CH, Donelan JM. Coordination of push-off and collision determine the mechanical work of step-to-step transitions when isolated from human walking. Gait Posture. 2012;35(2):292-297. doi:10.1016/j.gaitpost.2011.09.102.
- Huang T -w. P, Shorter KA, Adamczyk PG, Kuo AD. Mechanical and energetic consequences of reduced ankle plantarflexion in human walking. J Exp Biol. 2015:3541-3550. doi:10.1242/jeb.113910.
- Mahon CE, Farris DJ, Sawicki GS, Lewek MD. Individual limb mechanical analysis of gait following stroke. J Biomech. 2015;48(6):984-989. doi:10.1016/j.jbiomech.2015.02.006.
- Duncan PW, Sullivan KJ, Behrman AL, et al. Protocol for the Locomotor Experience Applied Post-stroke (LEAPS) trial: a randomized controlled trial. BMC Neurol. 2007;7(c):39. doi:10.1186/1471-2377-7-39.
- Reisman DS, McLean H, Keller J, Danks K a., Bastian a. J. Repeated Split-Belt Treadmill Training Improves Poststroke Step Length Asymmetry. Neurorehabil Neural Repair. 2013;27(5):460-468. doi:10.1177/1545968312474118.
- Helm EE, Reisman DS. The Split-Belt Walking Paradigm. Exploring Motor Learning and Spatiotemporal Asymmetry Poststroke. Phys Med Rehabil Clin N Am. 2015;26(4):703-713. doi:10.1016/j.pmr.2015.06.010.
- Wutzke CJ, Faldowski RA, Lewek MD. Individuals Poststroke Do Not Perceive Their Spatiotemporal Gait Asymmetries as Abnormal. Phys Ther. 2015;95(9):1244-1253. doi:10.2522/ptj.20140482.
- Donelan JM, Kram R, Kuo AD. Simultaneous positive and negative external mechanical work in human walking. J Biomech. 2002;35(1):117-124. doi:10.1016/S0021-9290(01)00169-5.
- Gottschall JS, Kram R. Energy cost and muscular activity required for leg swing during walking. J Appl Physiol. 2005;99(1):23-30. doi:10.1152/japplphysiol.01190.2004.
- Murray M, Hardee A, Goldberg RL, Lewek MD. Loading and knee flexion after stroke: Less does not equal more. J Electromyogr Kinesiol. 2014;24(1):172-177. doi:10.1016/j.jelekin.2013.10.006.
- Takahashi KZ, Lewek MD, Sawicki GS. A neuromechanics-based powered ankle exoskeleton to assist walking post-stroke: a feasibility study. J Neuroeng Rehabil. 2015;12(1):23. doi:10.1186/s12984-015-0015-7.
- Kesar TM, Reisman DS, Perumal R, et al. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture. 2011;33(2):309-313. doi:10.1016/j.gaitpost.2010.11.019.
- Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking Speed and Step Length Asymmetry Modify the Energy Cost of Walking After Stroke. Neurorehabil Neural Repair. 2015;29(5):416-423. doi:10.1177/1545968314552528.
- Kramers De Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Jt Surg – Ser A. 1996;78(10):1506-1514. http://www.scopus.com/inward/record.url?eid=2-s2.0-10244235407&partnerID=tZOtx3y1.
- Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture. 2003;18(1):114-125. doi:10.1016/S0966-6362(02)00165-0.
- Peterson CL, Cheng J, Kautz SA, Neptune RR. Leg extension is an important predictor of paretic leg propulsion in hemiparetic walking. Gait Posture. 2010;32(4):451-456. doi:10.1016/j.gaitpost.2010.06.014.
- Yen S-C, Schmit BD, Wu M. Using swing resistance and assistance to improve gait symmetry in individuals post-stroke. Hum Mov Sci. 2015;42:212-224. doi:10.1016/j.humov.2015.05.010.

4 Responses to “ALTERATIONS IN KINEMATICS AND ENERGY COST OF WALKING IN HEALTHY AND HEMIPARETIC GAIT USING FORCES APPLIED TO THE CENTER OF MASS”
Debby Givens
Kelly:
I enjoyed reading about your project. It looks like it was a tremendous amount of work to collect that many subjects under 3 conditions! And, analyzing the data, whew!
So, my question is… how did you determine the CoM location for each subject? And, which locations are you referring to – the sagittal, frontal, or coronal plane?
Kelly Hewitt
Hi Rob,
Thank you for your insight regarding our project! It certainly was a unique set-up, but definitely a feasible one in the clinic, as all it involved was a treadmill and theratubing. You brought up a great point though, the device we used to determine a 10% body weight pull is not commonly found in the clinic. The reason we chose a 10% pull is because it had been previously been determined to be the magnitude of horizontal aiding force required to elicit a decrease in COT. (from Gotschall, 2002) In other words, there is a “sweet spot” of pull force that is applied. That being said, it is fairly important to have just the right amount of pull force. However, the positive changes in COT that we saw here were elicited with a force applied during only the latter half of the gait cycle. It is possible that the timing of force application is more important than the amount of force applied. If this is the case and the set-up alone could elicit changes in COT, then it would very easily be a feasible clinical intervention. This is the exciting thing about this topic, is that there is so much more room for exploration!
Thanks again for your comments and questions!
Michael Lewek
Kelly
You did a wonderful job on this entire research project. This capstone is the culmination (so far) of all the hard work that you have done since the fall semester. Over the course of the past year you have spent countless hours learning to independently use just about every piece of equipment in the lab, test 20+ subjects, analyze and interpret the data, and present them in a clear and compelling manner. You should be proud of your efforts and your outcome. We will work to get this out for publication in the next few months and this will make an important contribution to the literature.
Well done.
BTW, I’m posting the same thing to Korre, since you two worked as such a great team together for the past year.
Mike
Rob Sykes
Great job, Kelly! Well written and intriguing findings! I think this study has exciting implications for clinical practice, and I am eager to read more about this topic. The novel device that you used to impose anterior and posterior forces is pretty ingenious. I hope future studies will replicate it in order to determine if the benefits observed during the anterior condition carry over to overground walking. Alternatively, could the difficultness of the posterior condition somehow lead to improvements during overground walking? I suppose the setup may not be terribly difficult to recreate in a clinical setting. Perhaps the most challenging part would be maintaining the 10% pull without having the necessary measuring equipment. How important do you think it is to maintain a pull force of 10%?
Again, great job!
-Rob