Created by: Cristina Raiti, SPT
For my capstone, I decided to share the project I worked on during my research elective in the 2016 fall semester. I utilized this research experience to further explore my interest in working with individuals with neurological disorders, specifically those who are post-stroke. I worked with Dr. Michael Lewek, PT, PhD to determine the capacity of the paretic limb during walking and to see if we could tap into the reserve capacity of the paretic limb to increase propulsive force. This was a single-session study that involved healthy, unimpaired participants as well as individuals who are post-stroke walking on a treadmill at their comfortable treadmill speed with a posterior pull-force applied to their center of mass for about 20 seconds at 0-10% of the participants’ body weight in 2.5% increments.
In addition to starting my research experience in the fall I began expanding my knowledge on the current literature by focusing on my critically appraised topic on the effect of a posterior pull-force at the center of mass during treadmill walking. My abstract was selected for a poster presentation at the UNC’s Human Movement Science and Biomechanics Research Symposium in March 2017. With feedback that I received and further analysis of collected data, I plan to complete and submit a manuscript for publication later this year.
This capstone would not have been possible without the invaluable guidance from my adviser, Dr. Michael Lewek, PT, PhD and research partner, Amanda Doty, SPT. In addition, my committee members, Dr. Vicki Mercer, PT, PhD and Marcie Brown, DPT, NCS, have provided me with feedback on both the wording, formatting and content on my partial manuscript and poster presentation and provided me with future concepts to consider in finalizing my manuscript as well as for this project.
ABSTRACT
1Cristina Raiti, 1Amanda Doty, 1,2Michael Lewek
1Division of Physical Therapy, University of North Carolina at Chapel Hill
2Human Movement Science Curriculum, University of North Carolina at Chapel Hill
Introduction: Individuals with hemiparesis following stroke are limited by slow, asymmetrical gait, and limited walking endurance. Post-stroke gait rehabilitation primarily focuses on improving gait speed, which is a challenge due to the limited ability to generate adequate propulsive force on the paretic side. Propulsive limb force is essential for propelling the body forward during gait. Current evidence suggests that individuals who are post-stroke may have the ability to generate greater propulsive forces to increase their walking speed. However, it remains unclear how much additional propulsive reserve exists during gait in the post-stroke population.
Objective: The purpose of this study was to determine the capacity in the paretic limb and to see if individuals post-stroke can scale the propulsive force appropriately on the paretic side.
Methods: This was a single session study, where kinetic and kinematic data were collected from six participants with chronic stroke (>6 months post-stroke) and 9 unimpaired subjects, using a dual-belt treadmill with force plates and an 8-camera motion capture system. We examined the immediate effects of an applied posterior pull-force at the pelvis. Participants walked on the treadmill at their comfortable walking speed and had a posterior pull-force (0 – 10% of the participants’ body weight in 2.5% increments) applied to the pelvis for ~20 seconds. The posterior pull-force was then removed in a descending fashion utilizing the same body weight percentages. Max pull force, peak paretic propulsion, propulsive impulse, peak plantar flexion moment and trailing limb angle at toe-off were collected from each participant. Given the preliminary nature of this study and small sample size, data analysis is qualitative in nature.
Results: As the posterior pull-force increased, the peak paretic propulsive force increased, in both the stroke and unimpaired groups up through the 10%BW condition. Plantarflexor moment and peak propulsion increased in both the chronic stroke and unimpaired groups with increased posterior pull-force. The absolute increase in the paretic propulsion was comparable between groups. Providing a posterior pull-force at the center of mass for short bouts has the potential to increase ankle joint moments in individuals post-stroke. The posterior pull-force was able to tap into the reserve capacity of post-stroke individuals. Plantar flexion moments tended to increase with increased posterior pull-force for the unimpaired participants, with peak plantarflexion moment varying in the post-stroke participants. Trailing limb angles were inconsistent.
Conclusions: Post-stroke individuals appear to be able to increase their peak propulsive force and propulsive impulse on demand during gait. Although we did not record EMG, providing a posterior pull-force at the center of mass for short bouts has the potential to increase muscle activity, and tap into the reserve capacity of post-stroke individuals. Further studies are required to determine the potential utilization of posterior pull-force for its adaptive effects to be utilized for post-stroke, gait rehabilitation.
INTRODUCTION
Individuals who have hemiparesis following a stroke are limited by slow, asymmetrical gait, and limited endurance.1 Slow gait speed is due, in part, to the inability to generate adequate propulsive force on their paretic side, which is essential for propelling the body forward during gait.2,3 Post-stroke gait rehabilitation primarily focuses on improving gait speed because it is a strong indicator of functional mobility and quality of life.4
There is evidence that suggests that paretic propulsion is modifiable and that individuals who are post-stroke have a reserve force that can generate greater propulsive forces to increase their walking speed.5,6 Reduced propulsive force during push-off is also seen in older adults; however, it has been found that older adults are able to significantly increased their propulsive forces and muscle activity during push-off when provided propulsive feedback, suggesting that there is an underutilized propulsive reserve that is available during gait.7 In addition to the potential for a reserve force capacity, individuals post-stroke are able to generate greater propulsive forces with similar amount of bilateral effort to achieve faster walking speeds and improve gait symmetry.6 Multiple studies have shown that an increase in generated propulsive force of the affected limb during gait is accompanied with improved gait performance.1,8,9
Compensatory strategies during gait, are common rehabilitation challenges that occur during gait training which limits improvements in functional mobility.9 After a stroke, it is common to see plantar flexor weakness on the paretic side which negatively affects limb propulsion,10 and results in compensations from other joints, such as the hip, to propel the limb and center of mass forward during gait.11 Individuals who are post-stroke typically utilize the hip flexors and trailing limb angle to advance the paretic limb from stance phase into swing phase.12,13,14 While increasing the trailing limb angle is required to restore a normal walking pattern, ankle moment is needed to increase propulsive force for more energy efficient gait.5 In addition to compensate for reduces paretic limb propulsion, the non-paretic limb may have earlier or greater power production initiation during leading limb’s double-support phase.10 Individuals who are post-stroke may take longer steps with the paretic limb which is correlated with negative work production during heel stroke, causing a step length asymmetry.10 Therefore, both power production of the non-paretic limb as well as step length asymmetry may contribute to the overall asymmetrical, slow gait speed commonly observed post-stroke.
There are studies that have successfully utilized restraining forces to increase propulsive force on healthy, unimpaired individuals.15 There is currently no available evidence in the literature that aims to investigate the amount of posterior pull-force needed to optimize propulsive force during gait in the post-stroke population. Although it is currently unknown if the reserve force can be utilized to increase propulsive force by providing a posterior pull to the pelvis during gait, this concept has important clinical implications for those with decreased propulsive force post-stroke, to assist with restoring normal gait.
The purpose of this study is to determine the ability of individuals post-stroke to immediately increase propulsive force. This particularly important because paretic propulsive force is already considerably lower than that of unimpaired individuals. Given that past studies have been successful in inducing an increase in paretic limb muscle activation and biomechanical output through altering gait,9,16 it is hypothesized that an increase in posterior pull force will generate an increase in propulsive force on the paretic side during the stance phase of walking, therefore increasing propulsive symmetry.
METHODS
Participants
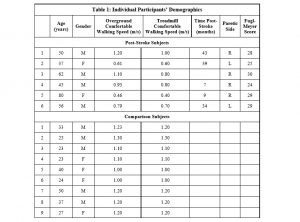
Data from six individuals (males, n=4; mean age = 55 ± 15 years; paretic limb: R=4, L=2; mean treadmill speed = 0.72 ± 0.2 m/s, mean overground walking speed = 0.78 ± 0.3 m/s, average Fugl Meyer scores = 27.5 ± 2.4) who presented with chronic hemiparesis (time post-stroke 26.4 ±17 months), and met the inclusion/exclusion criteria were recruited for testing. Inclusion criteria included: unilateral brain lesion due to stroke; ability to walk ≥5 minutes on treadmill with only hand hold assist for balance; ability to walk ≥ 10m overground without an assistive device. Exclusion criteria included: requiring an ankle-foot orthosis for ambulation; musculoskeletal, cardiorespiratory, metabolic, or other neurological disorder that could interfere with gait. Data from 9 comparison participants (males, n=4; age = 29.1 ± 6.3 years; mean treadmill speed = 1.15 ± 0.1 m/s) without any impairments, comorbidities, or conditions that affected their gait participated in the study. The study procedures were approved by the Institutional Review Board of the University of North Carolina.
Data Collection
Prior to data collection, each participant in the stroke group had their sensorimotor function evaluated using the Lower Extremity Fugl-Meyer. The fast and comfortable overground walking speeds were assessed utilizing two passes on the Zeno Walkway (ProtoKinetics, Havertown, PA). All participants had 38 reflective markers attached to anatomical landmarks and were captured using an 8-camera motion capture system similar (Vicon, Denver, CO) to that used by Murray et al.17 Participants wore a harness and were attached to an overhead system for safety, although no body weight support was provided.
 Participants walked on an instrumented dual-belt treadmill (Bertec, Worthington, OH) at their comfortable treadmill walking speed. While walking, a posterior restraining force was applied to the pelvis to approximate the body’s COM.18 The restraining force was provided via a stretched elastic element (TheraTubing) attached to a gait belt positioned around the pelvis. The gait belt provided an attachment site for the posterior pull force, which was monitored using a tension-load cell (MLP-150; Transducer Technique, Temecula, CA). Loads ranged from 0% to 10% of the participants body weight, in 2.5% increments. At each force level, the posterior force was applied for ~20 seconds in a step-like fashion. The pull-force was then removed in a descending step function utilizing the same body weight percentages. While walking, we recorded the ground reaction forces and pull force at 1200 Hz, and marker trajectories using the Vicon system at 120 Hz. Participants were allowed to use a unilateral handrail but were encouraged not to.
Participants walked on an instrumented dual-belt treadmill (Bertec, Worthington, OH) at their comfortable treadmill walking speed. While walking, a posterior restraining force was applied to the pelvis to approximate the body’s COM.18 The restraining force was provided via a stretched elastic element (TheraTubing) attached to a gait belt positioned around the pelvis. The gait belt provided an attachment site for the posterior pull force, which was monitored using a tension-load cell (MLP-150; Transducer Technique, Temecula, CA). Loads ranged from 0% to 10% of the participants body weight, in 2.5% increments. At each force level, the posterior force was applied for ~20 seconds in a step-like fashion. The pull-force was then removed in a descending step function utilizing the same body weight percentages. While walking, we recorded the ground reaction forces and pull force at 1200 Hz, and marker trajectories using the Vicon system at 120 Hz. Participants were allowed to use a unilateral handrail but were encouraged not to.
Data Management
Max pull force, peak paretic propulsion, propulsive impulse, peak plantar flexion moment and trailing limb angle at toe-off were collected from each participant. The data collected was time normalized to the gait cycle and then averaged across each condition.
Peak propulsion was expressed by calculating the peak of the anteriorly directed ground reaction force from the dual-belt treadmill. Propulsive impulse is the integral of the anterior ground reaction force. Plantar flexion moment was calculated using Visual 3D (C-Motion Corp) using inversion dynamics and the peak was chosen. The trailing limb angle at toe-off represents the angle of a vector from the pelvis center of mass and the center of pressure with a vector from the pelvis center of mass straight down.
Data Analysis
Ground reaction force and kinematic data were low-pass filtered (20Hz and 6Hz, respectively) with a fourth order Butterworth filter. Peak propulsion was expressed by calculating the peak of anterior force from the dual-belt treadmill. Plantar flexion moment was calculated using Visual 3D process of inversion dynamics. Trailing limb angle was measured through the pelvic center of mass angle and center of pressure from foot ground reaction force.
Data were analyzed using SPSS (ver 24). Specifically, we used a repeated measures ANOVA (repeated for pulling force magnitude) to determine differences between groups and between the different magnitudes of pull force. Post-hoc tests were performed for significant main effects or interaction effects. We used ANOVAs and paired samples t-tests for post-hoc analyses.
Results
This is an ongoing process. We are continuing to collect, and analyze data, so the results are incomplete at this time.
References
- von Schroeder HP et al. Gait parameters following stroke: a practical assessment. Rehabil Res Dev. 1995;32(1);25-31. http://www.ncbi.nlm.nih.gov/pubmed/7760264.Accessed March 25, 2017.
- Peterson CL, Hall AL, Kautz SA, Neptune RR. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J Biomech 2010;43(12):2348-2355. doi:10.1016/j.jbiomech.2010.04.027.
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke 2006;37(3):872-876. doi:10.1161/01.STR.0000204063.75779.8d.
- Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke 2007;38(7):2096-2100. doi:10.1161/STROKEAHA.106.475921.
- Hsiao H, Knarr BA, Higginson JS, Binder-Macleod SA. Mechanisms to increase propulsive force for individuals poststroke. J Neuroeng Rehabil 2015;12:40. doi:10.1186/s12984-015-0030-8.
- Hurt CP, Wang J, Capo-Lugo CE, Brown DA. Effect of progressive horizontal resistive force on the comfortable walking speed of individuals post-stroke. J Neuroeng Rehabil 2015;12:12. doi:10.1186/s12984-015-0007-7.
- Franz JR, Maletis M, Kram R. Real-time feedback enhances forward propulsion during walking in old adults. Clin Biomech (Bristol, Avon) 2014;29(1):68-74. doi:10.1016/j.clinbiomech.2013.10.018.
- Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA. Targeting paretic propulsion to improve poststroke walking function: a preliminary study. Arch Phys Med Rehabil 2014;95(5):840-848. doi:10.1016/j.apmr.2013.12.012.
- Clark DJ, Neptune RR, Behrman AL, Kautz SA. Locomotor Adaptability Task Promotes Intense and Task-Appropriate Output From the Paretic Leg During Walking. Arch Phys Med Rehabil 2016;97(3):493-496. doi:10.1016/j.apmr.2015.10.081.
- Mahon CE, Farris DJ, Sawicki GS, Lewek MD. Individual limb mechanical analysis of gait following stroke. J Biomech 2015;48(6):984-989. doi:10.1016/j.jbiomech.2015.02.006.
- Phadke CP. Immediate effects of a single inclined treadmill walking session on level ground walking in individuals after stroke. Am J Phys Med Rehabil 2012;91(4):337-345. doi:10.1097/PHM.0b013e31823cabe3.
- Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. Phys Ther 1994;74(9):872-885. doi:10.1093/ptj/74.9.872.
- Olney SJ, Griffin MP, Monga TN, McBride ID. Work and power in gait of stroke patients. Arch Phys Med Rehabil 1991;72(5):309-314.
- Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch Phys Med Rehabil 2007;88(9):1127-1135. doi:10.1016/j.apmr.2007.05.027.
- Gottschall JS, Kram R. Energy cost and muscular activity required for leg swing during walking. J Appl Physiol 2005;99(1):23-30. doi:10.1152/japplphysiol.01190.2004.
- Regnaux JP, Pradon D, Roche N, Robertson J, Bussel B, Dobkin B. Effects of loading the unaffected limb for one session of locomotor training on laboratory measures of gait in stroke. Clin Biomech (Bristol, Avon) 2008;23(6):762-768. doi:10.1016/j.clinbiomech.2008.01.011.
- Murray M, Hardee A, Goldberg RL, Lewek MD. Loading and knee flexion after stroke: Less does not equal more. J Electromyogr Kinesiol 2014;24(1):172-177. doi:10.1016/j.jelekin.2013.10.006.
- Gottschall JS, Kram R. Energy cost and muscular activity required for leg swing during walking. J Appl Physiol 2005;99(1):23-30. doi:10.1152/japplphysiol.01190.2004.


6 Responses to “STROKE SURVIVORS DISPLAY PROPULSIVE RESERVE IN THEIR PARETIC LIMB DURING GAIT”
Mike Lewek
Cristina
Nice job on this project. It was a pleasure to work with both you and Amanda this year. You took a technically challenging project and were able to independently collect data from multiple sources. These data are valuable for showing the capabilities of individuals post-stroke. Well done (I’m copy/pasting the same comments to Amanda since you worked so well together).
Mike
Cristina Raiti
Thank you, Mike! I had a great time not only interacting with the patient population that I enjoy working with, but also in learning about what goes into the research process. This was a very rewarding project that I am excited to continue working on. Thank you for being a great adviser and helping Amanda and I get through the many challenges that we faced.
Cristina
Vicki Mercer
Hi Cristina-
Interesting study! Glad to see that patients with stroke were able to scale their paretic limb propulsive forces with changes in posterior forces applied at the pelvis. How did you increase the resistance applied through the theratubing? Simply by having a researcher pull harder (and monitoring force using the load cell)? Or did you hook the end of the theratubing to a pole or other object and move it posteriorly (further away from the patient) until the desired amount of force was obtained?
Again, great work on this project!
Best,
Vicki
Cristina Raiti
Hi Vicki,
Thank you. I am hoping that this study will provide a basis for many more studies to expand from, hopefully into an intervention that can be used in the clinic. We increased the resistance by having one of the researchers (usually me) pull the theratube backwards harder, while Amanda would monitor the force on the load cell through the graph on the computer. Our communication was great and we were able to maintain a constant pull-force, although some times participants would “drift” further back on the treadmill when they were getting fatigued and we were sure to adjust for that quickly by Amanda providing me with feedback.
Best,
Cristina
Lauren Kozar
Cristina,
I was very intrigued to read about your study after completing my final stroke assignment for our Advanced Patient Management II Course. During this assignment we were asked to develop intervention strategies to improve the lower extremity strength and endurance in a patient with chronic stroke so he could become a community ambulatory. After countless hours of research, I noticed that there is a significant need for research regarding the improvement of walking in the chronic stroke population. Thus, your project is extremely important in helping to fill this void in chronic stroke research, while also being the first study to investigate the amount of posterior pull-force needed to tap into the energy reserves of the paretic limb in order to optimize propulsive force during gait in the post-stroke population.
Overall, I think you did an excellent job on your project. Not only did you do a great job summarizing related research in your introduction, but you used a lot of recent evidence to justify your project. That being said, what I am most impressed with is your methods section. You provided enough detail that someone could easily reproduce this study in the future, and I hope they do so we can determine the potential utilization of this intervention strategy in post-stroke gait rehabilitation. Your poster was also very well constructed. It was easy to follow, well-organized, contained only the necessary information so it as not to word heavy, and the graphs and pictures were the perfect size to be seen by the audience.
I only have two questions regarding your project: (1) When you say you are trying to determine the “capacity” of the paretic limb do you mean how much reserved energy they having in their paretic limb? and (2) How did you select 20 seconds as the time interval for data collection at each application of posterior force?
Again, you did an excellent job, and I look forward to reading the completed paper!! Congrats on submitting your capstone!
Cristina Raiti
Lauren,
Thank you for your feedback. You are right on track, there is little research out there on how to improve walking in chronic stroke patients, and I am hoping that my research (as well as Amanda’s) will provide a basis for others to expand on (looking at long-term effects, carry over, standardized intervention). I am glad you enjoyed my project, I worked very hard on it and have been working on it since the Fall semester.
To answer your first question, in short, yes. We know that there is a reserve capacity of the paretic limb, but we don’t know how much there is or how much we have to make that side work to get the full reserve force out of there. Questions to consider when thinking about the reserve force are: if we pull a little will we get only a little increased force production? How much do we need to pull until we see a plateau? How close to the non-paretic side can it get to? We did not address all of these questions in this study, but they are questions that should be asked. We were simply looking at if pulling them backwards at their center of mass would allow them to utilize their reserve force and if the reserve force would increase with an increased pull-force, and if they are generating more force on the paretic side, where is it coming from? (plantarflexors, hip?). We selected 20 seconds because I was just looking at the immediate effects of the pull-force (if you are interested in the adaptive effects of the pull-force I encourage you to look at Amanda Doty’s project). Also, each participant had to do my portion of the study and then walk on the treadmill for 14 minutes (10 of that with a constant pull-force) so patient fatigue was a factor as well.
Again, thank you for your feedback,
Cristina