Understanding the Multifactorial Causes of Falls in Individuals Post-Stroke
By Kristen Shumaker, SPT and Kristen Massey, SPT
Background
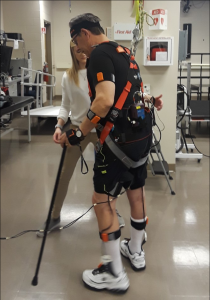
Both of us have an interest in the stroke population as well as the biomechanical and psychosocial consequences of stroke. We also both had experience conducting research projects prior to PT school and were interested in continuing to pursue this in our graduate careers. Thus, we were excited to read about Dr. Lewek’s research project regarding the causes of trips and trip-related falls during walking in the stroke population. This research will hopefully lead to an algorithm that can accurately predict trips and will contribute to the development of an intervention that can potentially prevent the trips altogether.
Testing took place at Dr. Lewek’s lab at UNC and Dr. Huang’s lab at North Carolina State University. Subjects completed 3 separate sessions, including baseline testing, treadmill walking, and over ground walking. During walking sessions, participants walked (on treadmill or over ground) while we attempted to elicit a trip via non-environmental distractors, including changing gait speed, directional gaze changes, and cognitive tasks.
We created a poster to present at Human Movement Science Research Symposium (HMSC) in March; however, COVID-19 had other plans. We intended to present the significance of our research and our initial findings regarding the distractors that seemed to induce the most trips in our participants at that time in the study (i.e. divided attention, head turns, fatigue).
The purpose of this capstone (review paper) is to present the various causes of falls, the consequences of falls, and how we as clinicians can intervene to prevent them.
Acknowledgements
Dr. Michael Lewek, PT, PhD – We are so grateful for your expertise, your teaching, and your patience as you led us through this entire process. We appreciated this incredible opportunity to advance our critical thinking, clinical skills, and scientific writing. We look forward to applying this in our future careers. Thanks for all of your support!
Dr. Vicki Mercer, PT, PhD & Dr. CJ Hamilton, PT, DPT – Thank you both for investing your time in our capstone project and serving as committee members. By sharing your research and clinical expertise in this area, we were able to make a product of which we are proud.
Abstract
Individuals recovering from stroke are at a high risk for falls in all stages and settings post-stroke. These falls lead to physical and psychosocial consequences that greatly impact function and quality of life. The consequences of falls in this population can include fractures, chronic disability, depression, loss of independence, and fear of falling. The aim of this review paper is to outline the mechanisms of falls post-stroke to determine potential areas for future clinical intervention. Most falls occur during mobility tasks, such as walking and transfers; and can result from impaired postural control, reduced muscular force generating capacity, and biomechanical factors that reduce the ability to respond appropriately to internally or externally generated perturbations. The cause of falls is multifactorial and can be attributed to a combination of intrinsic factors (originating from the individual) or extrinsic factors (components of the environment with which the individual interacts). Because post-stroke falls are more commonly associated with intrinsic risk factors, this suggests the potential for clinical interventions to help reduce these deficits and resulting fall risk.
Introduction
Individuals following a stroke are at a considerably higher risk of falling compared to unimpaired, age-matched individuals.1 With 0.65-0.85 more falls per person per year in those recovering from stroke versus the general population,2 most studies report that >70% of individuals fall after stroke.3–5 These falls occur in all clinical settings, across all stages of recovery (i.e., chronicity) including, acute, inpatient rehabilitation, and community outpatient settings. Falls are the leading cause of impaired functional independence and reduced community mobility for individuals with stroke because of the physical and psychosocial consequences.6–8 Given the burden of stroke, and the increased burden of a subsequent fall, it is critical to understand what factors contribute to falls in individuals following stroke. This review will examine the epidemiology around falls across all levels of chronicity. Furthermore, we seek to provide clarity on the most common factors that contribute to falls for individuals recovering from stroke. Specifically, we will examine both intrinsic and extrinsic factors to determine how they might influence the risk of falls. Through this review, we hope to provide insight into how rehabilitation professionals can best influence these factors that most contribute to falls.
Epidemiology of Falls Post-Stroke
Compared to their age-matched controls, individuals following a stroke are at an increased risk for falls.1 Although many falls occur within the first 6 months after stroke,5,8–11 individuals with stroke remain at an increased risk for falls from the acute to chronic phase.6,10 Indeed, during inpatient rehabilitation, 14% to 65% of people with stroke experience one or more falls,9,12 with the risk for falls in the hospital after an acute stroke being more than double that of falls in the hospital for pathologies such as congestive heart failure and community-acquired pneumonia.13 After discharge, the proportion of reported falls in community-dwelling stroke survivors ranges from 40% to 73% and from 43% to 70% for a 6-month and 1 year follow-up respectively.1,9,14–23
Consequences of Falls
The consequences of falls after stroke can cause major medical complications, such as fractures, hematomas, and concussions that can remain a major health concern through post-stroke life. Falls after stroke are particularly troubling due to the compounding levels of debility that yield worse rehabilitation outcomes, loss of independence, and chronic disability.24 As the leading cause of impaired functional independence and diminished community mobility for individuals with stroke, falls can have devastating physical and psychosocial consequences.6–8
Between 28-72% of people with chronic stroke who fall report a resulting injury.3,20 Injuries are more common in those with stroke due to the stroke-induced hemiparesis, slower reflexive responses, and inability to recover from loss of balance. The inability to appropriately respond to a significant loss of balance can be attributed to deficits in the hemiparetic arm, as well as the need for an assistive device in the non-paretic hand, leaving people unable to provide a suitable protective response with either arm. While many injuries from a fall can be classified as mild (i.e. bruises, lacerations), some falls can result in a fracture, as individuals with stroke have a sevenfold increase in fracture risk compared to unimpaired individuals.3,25 In fact, 45-59% of fractures in people with stroke occur at the hip, with fracture of the paretic hip being twice as likely as a hip fracture following a fall in an individual without stroke.3,5 After experiencing a hip fracture, individuals post stroke may not regain independent mobility, leading to increased mortality.10,26
Falls in those recovering from stroke can also have substantial psychosocial consequences. Fear of falling is common after stroke because of the high fall rate.4 A fear of falling can reduce mobility, which may create a vicious cycle that generates further psychosocial consequences. For example, many individuals participate in fewer meaningful life activities because they have a fear of falling,4 which, in turn may contribute to social deprivation and a loss of independence.7 To make matters worse, falls in individuals with stroke often lead to depression.5 It is important to note that Post Stroke Depression (PSD) is the most common psychological consequence following a stroke.27 Prevalence of PSD ranges between 20 and 65 percent, depending on the population and definition used.28 Thus, an increased risk of depression resulting from falls after stroke compounds this already prevalent condition of PSD. Because depression acts as both a risk factor and a consequence of falls,5 this relationship can become cyclical.
Circumstances around Falls
Falls after stroke typically occur during mobility tasks, such as walking, transfers, negotiating stairs, or other activities of daily living (ADLs).3,9,26 For individuals recovering from acute stroke, falls in the hospital most commonly occur during walking, such as when individuals lose their balance, get their foot “stuck,” or have difficulty performing a transfer.1 Most falls post-stroke that occur in inpatient rehabilitation occur in a patient’s room or bathroom when completing a transfer or changing position.29 Falls are especially common when patients act against the instruction of healthcare personnel, such as walking or transferring without the recommended supervision or assistive device.29,30
Although falls in the acute phase of recovery from stroke most often occur during walking or transfers,9 in community-dwelling (chronic) stroke survivors, walking is the most common activity leading to a fall.4,25 For community ambulators, falls during walking often result from turning, dragging a foot, tripping, or slipping.9 Notably, many walking related falls in the chronic phase may arise from environmental perturbations, such as slips and trips.6 In addition to walking, falls can also occur during transfers and performance of ADLs including toileting, gardening, and community activities.3 Individuals post-stroke often have difficulty with position changes, standing up or sitting down, or transferring between surfaces,9,29 all of which create opportunities to lose focus or lose balance and fall. Age, history of falls, number of prior strokes, use of medications, poor cognition, and abnormal mood have all been associated with increased falls in the chronic phase of recovery.8 Individuals with chronic stroke often continue to demonstrate balance and gait deficits consistent with greater standing sway, impulsivity, slowed response time, and reduced force generation during sit-to-stand.9 Importantly, these impairments are also associated with falls during mobility tasks, such as walking, transfers, and performing ADLs.
Factors Contributing to Falls
Falls often can be attributed to factors originating from the individual (i.e., intrinsic factors), from the individual’s interactions with the environment (i.e., extrinsic factors), or from a combination of both intrinsic and extrinsic factors. Extrinsically-generated falls may be due to obstacles such as loose carpet or to large external forces placed on the body, such as when opening a heavy door.12,29 Regardless of the source, extrinsically-generated falls result from an altered environment that leads to a disruption in the location of the center of mass (COM) relative to the stable base of support (BOS).12 In contrast, intrinsically-generated falls are the result of self-induced perturbations in which voluntary and/or involuntary forces from within the body are abnormal.12 Examples can include impaired postural sway, instability due to the legs giving way, reduced force generating capacity of the muscles, visual impairment, or even cognitive impairment (perceptual error, sensory distraction, or inattention).10
Evidence suggests that falls post-stroke are more commonly associated with intrinsic versus extrinsic risk factors, regardless of setting or time post stroke.20,29,31 The most common intrinsic risk factors include impairments in strength (due to the resulting hemiparesis), postural stability, cognition (i.e. distraction, inattention, perceptual error), and altered motor control that influences balance.29,31 Falls associated with extrinsic factors such as those that occur when leaning on an unstable object or tripping over an obstacle are not as common after stroke as falls associated with intrinsic factors.29 It is suspected that extrinsically-generated falls are particularly less frequent in the acute and subacute environment because of the supervision from nurses or therapists,29 the well-lit, level floors, and open areas with few obstacles or obstructions. Thus, there are fewer opportunities to be influenced by external perturbations, and when opportunities for external perturbations do exist, there are clinicians around for supervision and safety. As individuals post-stroke progress into the chronic stage and become community ambulators, falls associated with intrinsic factors remain more prevalent, and occur most often during walking.9
Responding to Balance Perturbations
The strategies to recover from intrinsically or extrinsically generated perturbations are often impaired after stroke. External perturbations that challenge postural stability require quick sensorimotor feedback mediated reactions, placing a large reliance on sensory feedback mechanisms.12,32,33 This involves gauging a disturbance and then generating a control signal that counteracts that disturbance.12,32,33 In contrast, self-induced perturbations during voluntary movements require intact feedforward responses to minimize the perturbation in the first place to maintain equilibrium. Such feedforward mechanisms may be impaired at the nervous system level (i.e., lack of appropriate control signal) or at the peripheral musculoskeletal level (i.e., inability to produce the necessary movement).12
After stroke, delayed and uncoordinated responses to intrinsically and extrinsically generated balance perturbations can lead to falls. A major factor that determines the occurrence of a fall is the ability to react to a loss of balance.2 However, individuals with stroke have impaired reactive balance control, which is marked by a delayed and smaller amplitude postural response compared to healthy controls.34 Individuals post-stroke typically prefer to step with their nonparetic limb when a step is taken to prevent a fall and may use a hopping strategy in order to avoid bearing weight through the paretic limb. This strategy is generally characterized by shorter step lengths and more trunk rotation, which results in a less stable foot placement.35 The resulting ineffective reactions to losses of balance yield a reduced ability to recover from either an intrinsically- or extrinsically-generated perturbation to prevent a fall.2,5,36,37
A delayed reactive response is associated with a less efficient recovery pattern. When responding to a trip, people with chronic stroke have a decreased ability to arrest and reverse trunk flexion and execute a sufficiently long first step in response to the perturbation, which is necessary for successful trip recovery.38 Such deficits in reactive stepping patterns occur because of impairments in limb control and coordination.39 The impaired reactions to a postural perturbation, therefore, often present as an abnormal stepping strategy to try to recover balance, which only increases the risk for a fall.2,5,39 The implementation of such an inefficient stepping response occurs commonly in response to large-magnitude perturbations during stance and during dynamic activities including gait.6 As a result, individuals following stroke exhibit a delayed step initiation, decreased step length, and more steps to recover balance from a perturbation when compared to unimpaired controls.6 Walking requires well-coordinated timing and placement of steps to control for the small perturbations of the trajectory of COM, demonstrating (reactive and/or anticipatory) balance control. Individuals with stroke demonstrate large amounts of variability in step length, step time, and step width.34 When this typical gait variability is paired with the less predictable environment in a community-ambulating stroke survivor, it can lead to impaired balance control during walking and greater falls risk.34
Effect of Muscle Force Generating Capacity
Although neural control deficits are implicated in the high incidence of falls post-stroke, the presence of muscle impairments also contributes to falls. The force generating capacity of affected muscles is already reduced after stroke (i.e., hemiparesis), and can be further compounded by the local muscle fatigue that occurs with prolonged activity. Muscle fatigue is defined as a “decrease in maximal force or power production in response to contractile activity,”40 and can arise from central or peripheral sources. Central fatigue is attributed to the central nervous system, which decreases the neural drive to the muscle, whereas peripheral fatigue is attributed to changes “at or distal to the neuromuscular junction.”40 Although both central fatigue (e.g., reduced voluntary ability to activate the muscle maximally) and peripheral fatigue (e.g., loss of force-generating capacity), are prevalent in the post-stroke population,41,42 individuals with chronic stroke tend to have faster onset of central fatigue in the paretic limb versus the non-paretic limb.40,43 In fact, muscle fatigue is a common symptom in patients with chronic stroke, representing a substantial contributor to impaired daily activities. Persistent fatigue may be compounded by deconditioning arising from decreased activity,44 and the increased energy cost attributed to motor impairments.45 These fatigue-related factors may increase the risk for falls after stroke46 by exacerbating limitations in gait and functional mobility.44
Impact of Fatigue on Gait Stability
Excessive muscle fatigue has been estimated to occur in up to 70% of patients recovering from stroke, which can be readily observed in functional situations that can lead to increased risk for falls.47 As an example, gait speed has been shown to decrease significantly after only six minutes of continuous effortful walking in 40% of individuals with chronic stroke.47,48 Although some individuals initially start with a fast walking speed during the 6MWT and decrease speed over the course of the test, others select a slower speed that they can maintain to cover the same total distance. Furthermore, the observation of slowing down during prolonged walking is apparent in both the subacute (< 3 months) and chronic phase (> 3 months) following stroke; although, this functional fatigue is somewhat lessened in the chronic phase.49,50 Importantly, these reductions in gait speed during prolonged walking may be indicative of real-world ambulatory activity and reflect underlying deficits of physical inactivity that may contribute to an increase in falls.48 In particular, the gradual alteration in gait during prolonged walking may have negative consequences for falls, and may explain, in part, why so many falls occur during walking.
Individuals who exhibit functional fatigue during prolonged walking consequently exhibit signs consistent with impairments that might contribute to falls.47 For example, walking speed reductions during the 6 MWT are correlated with reduced gait dynamic stability.47 Consequently, individuals following stroke who fatigue quickly tend to show decreased coordination the longer they walk, suggesting a possible association between onset of local muscle fatigue and an increased risk for falls.44,47 For example, when knee flexor and extensor muscle fatigue is induced prior to walking in individuals post-stroke, balance during gait is reduced as evidenced by greater anteroposterior and mediolateral postural sway, calculated from center of pressure displacement.51 Additionally, those who fatigue quicker during a 6MWT exhibit greater temporal gait asymmetry 50, which has been associated with reduced static and dynamic balance52 and may provide further evidence linking fatigue with gait-related falls after stroke.
Reduced force generating capacity (i.e., strength) is common following stroke and can negatively influence gait, the primary activity that leads to falls. In particular, individuals recovering from stroke often rely more on their hip flexors for limb advancement during gait, due to the presence of distal muscle weakness.53 Because the hip flexors are already compensating for the weak plantarflexors, additional force decline of the hip flexors due to fatigue leaves the paretic limb with fewer compensatory strategies.53 Consequently, the ability to respond to environmental demands becomes further limited. Reduced force from the hip flexors is particularly troubling due to the continued reliance on symmetric sub-maximal torque production to produce forward progression while maximizing postural stability during walking.54 Any resulting weakness in the paretic hip flexors, most often due to muscle atrophy, impaired voluntary activation and neuromuscular fatigue,54 limits the ability of the hip flexors to adequately raise the foot during swing to compensate for the diminished knee flexion and dorsiflexion.55 Without a means to increase limb flexion, people post stroke are at an increased risk of a trip-related fall during swing phase.
Biomechanical Factors contributing to Falls
Indeed, there are several biomechanical factors that contribute to gait related falls in individuals post stroke. Some of the most common gait deficits found in patients post stroke include reduced propulsion at push-off, decreased hip flexion, knee flexion and dorsiflexion during swing phase, and reduced stability during stance phase.56 Reduced stability during static standing is often associated with increased body sway in individuals post-stroke, as these individuals have been reported to have one and a half to five times the amount of sway as unimpaired controls.57–64 Furthermore, the reduced limb flexion during swing phase, leads to less foot clearance during swing phase and greater risk for trip-related falls.65,66 Reduced toe clearance during swing can be predicted late in stance from the sagittal plane orientation of the shank and the elevation velocity of the foot’s center of gravity.67 Such a prediction model gives hope that a timely intervention can be deployed to avoid the trip. Absent an intervention, the relative lack of limb flexion during swing is typically compensated for by stroke survivors with increased circumduction, pelvic tilt on the paretic side, and/or trunk lean toward the nonparetic side.68 When these compensations fail, however, the foot is more likely to make contact with the ground during swing phase, causing a trip-related fall. Decreased dorsiflexion and reduced knee extension may contribute to a forefoot landing. When combined with premature and excessive calf muscle activation, the forefoot strike pattern leads to a limb configuration that creates a smaller base of support and decreased stance stability.69,70
Multi-joint coupling may reduce limb flexion
There are several possible contributors to a reduction in limb flexion during walking for individuals following stroke. Cruz et al. note the presence of cross-planar multi-joint coupling between sagittal plane knee and frontal plane hip torques.71 The coupling of hip adduction and knee extension increases leg length. Consequently, this creates a need for compensatory strategies, such as hip hiking and circumduction, for toe clearance during swing phase to reduce the risk for tripping.71 Heteronymous stretch reflexes from the hip flexors to the knee extensors may also contribute to a decrease in swing phase knee flexion.72 As the hip extends during late stance, stretching the hip flexors, it appears there may be an increase in quadriceps activation, which is associated with reduced knee flexion during swing phase. The presence of abnormal synergies following stroke may therefore have negative functional consequences that impede the ability to achieve adequate toe clearance during swing, resulting in a higher likelihood of trip-related falls.72
Inadequate Obstacle Clearance Can Contribute to Falls
Environmental demands during gait require adaptability to maintain balance and reduce the risk of falling. As noted previously, the feedback response to collision with an obstacle provides important information about how individuals with chronic stroke avoid falls.38,73 But avoiding the environmental obstacle in the first place reduces the likelihood of falling. Unfortunately, individuals with chronic stroke have difficulty with obstacle avoidance due to an inability to shorten their stride, which results in the toe making contact with the obstacle and inducing a trip.38 Alternatively, the use of a long step strategy for stepping over an obstacle may prove challenging due to an inability to sufficiently lengthen the stride, leading to obstacle contact at the heel.38,74 Because we routinely encounter uneven surfaces or objects on the floor throughout the day, it is not surprising that many falls occur during walking.
There are several common mechanisms for individuals post-stroke that may contribute to the inability to adequately avoid an object on the ground during walking. For example, greater co-contraction between the tibialis anterior and medial gastrocnemius when performing obstacle crossing75–77 can reduce dorsiflexion range of motion. With less ankle dorsiflexion, there may be less toe clearance, which increases the risk for tripping. Furthermore, individuals with chronic stroke activate the tibialis anterior to a greater percentage than unimpaired controls, suggesting that people with chronic stroke face greater neuromuscular challenges with obstacle avoidance. These biomechanical and neuromuscular deficits require greater muscle activation, and thus may produce the secondary effect of fatigue onset during gait, which can compound the risk for falls.75
As a potential method for reducing unanticipated foot contact with ground-based objects while walking and subsequent tripping, gait training with real-time augmented toe clearance biofeedback can decrease tripping risk in those with chronic stroke. In particular, the provision of visual augmented feedback (toe vertical displacement) can successfully increase foot height.11,78–80 Rather than using joint-specific (i.e., knee or ankle minimum flexion angles) interventions, these findings suggest that targeting the end-effector may reduce the risk for tripping. Importantly, these findings also suggest that individuals post-stroke have the inherent capacity to create adequate limb clearance but may not use it on a regular basis. Therefore, when an unanticipated object is present, an individual with post-stroke hemiparesis is more likely to trip. Although extrinsic risk factors are not as commonly associated with falls as are intrinsic risk factors,20,29,31 they still have negative implications for mobility and participation as individuals avoid activities that may predispose them to a fall.
Mediolateral gait stability and falls
The mechanical state of the stance limb during gait influences the simultaneous swing phase hip abductor activity in the contralateral limb.4 As a result, people with chronic stroke place the paretic limb more laterally when compared to unimpaired controls.4 It is proposed that this lateral foot placement may arise from an inaccurate perception of the swing limb’s location relative to the center of mass (COM) or an inability to regulate the swing limb gluteus medius activity during gait.4 Instead of controlling foot placement location to move the COM toward midline with each step, individuals with hemiparesis appear to maintain gait stability by balancing their COM over the nonparetic limb while placing the paretic limb more laterally.4 The resulting lateral foot placement may therefore predispose individuals with stroke to reduced trip recovery and a subsequent fall during gait. In particular, an increase in lateral foot placement may increase frontal plane angular momentum due to an increased lever arm from the ground reaction force about the COM.81 Importantly, the increase in lateral foot placement and angular momentum are both related to decreased clinical balance measures, (e.g., Berg Balance Scale and DGI),81 providing additional support for their importance in fall prevention.
Conclusion
Falls post-stroke are very common and are often the consequence of an inability to respond to an internally or externally generated perturbation.35 Individuals post-stroke are more prone to falls associated with intrinsic risk factors such as inadequate limb flexion during swing, decreased toe clearance while walking, reduced postural stability, increased body sway during quiet standing, increased fatigue while walking, gait asymmetry, and ineffective recovery patterns after trips.35,50,57–64,72 Falls in individuals post-stroke tend to occur during mobility tasks such as walking, transfers, navigating stairs, and other ADLs.3,9,26 These findings suggest that clinicians can intervene to help reduce the risk of falls, given that these intrinsic factors can be addressed by various interventions to target each deficit. For example, Dean and Kautz have addressed gait instability through novel interventions targeting lateral stability.4 Additionally, Bhatt et al. have used slip-like perturbations during walking to assess the recovery response in individuals following stroke and the potential benefits of training-induced modulation of COM position and velocity during gait.82 Their results indicate that individuals post-stroke can improve their reactive balance with a perturbation training paradigm, which presents significant clinical importance.82 This growing number of interventions targeting dynamic stability during gait and postural tasks provides encouragement that clinicians will be able to substantially modify fall risk factors for individuals post-stroke. Clinicians serve a vital role in employing various methods to target the factors that contribute to trips and trip-related falls post-stroke, and they have the opportunity to prevent falls altogether. More importantly, by reducing falls post-stroke, clinicians can also reduce the subsequent consequences of these falls, including injury, disability, mobility deficits, depression, and loss of independence. Addressing falls risk allows clinicians to encourage safe, functional mobility and independence for individuals post-stroke.
Bibliography
- Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ. 1995;311(6997):83-86. doi:10.1136/bmj.311.6997.83
- de Kam D, Roelofs JMB, Bruijnes AKBD, Geurts ACH, Weerdesteyn V. The next step in understanding impaired reactive balance control in people with stroke: the role of defective early automatic postural responses. Neurorehabil Neural Repair. 2017;31(8):708-716. doi:10.1177/1545968317718267
- Schmid AA, Yaggi HK, Burrus N, et al. Circumstances and consequences of falls among people with chronic stroke. J Rehabil Res Dev. 2013;50(9):1277-1286. doi:10.1682/JRRD.2012.11.0215
- Dean JC, Kautz SA. Foot placement control and gait instability among people with stroke. J Rehabil Res Dev. 2015;52(5):577-590. doi:10.1682/JRRD.2014.09.0207
- Handelzalts S, Steinberg-Henn F, Levy S, Shani G, Soroker N, Melzer I. Insufficient balance recovery following unannounced external perturbations in persons with stroke. Neurorehabil Neural Repair. 2019;33(9):730-739. doi:10.1177/1545968319862565
- Salot P, Patel P, Bhatt T. Reactive Balance in Individuals With Chronic Stroke: Biomechanical Factors Related to Perturbation-Induced Backward Falling. Phys Ther. 2016;96(3):338-347. doi:10.2522/ptj.20150197
- Weerdesteyn V, de Niet M, van Duijnhoven HJR, Geurts ACH. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195-1213.
- Kerse N, Parag V, Feigin VL, et al. Falls after stroke: results from the Auckland Regional Community Stroke (ARCOS) Study, 2002 to 2003. Stroke. 2008;39(6):1890-1893. doi:10.1161/STROKEAHA.107.509885
- Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002;83(2):165-170. doi:10.1053/apmr.2002.28030
- Costa AG de S, Oliveira-Kumakura AR de S, Araujo TL de, Castro NB de, Silva VM da, Lopes MV de O. Stroke and risk factors for falls in elderly individuals. Rev Rene. 2017;18(5):663. doi:10.15253/2175-6783.2017000500014
- Begg RK, Tirosh O, Said CM, et al. Gait training with real-time augmented toe-ground clearance information decreases tripping risk in older adults and a person with chronic stroke. Front Hum Neurosci. 2014;8:243. doi:10.3389/fnhum.2014.00243
- Gray VL, Yang C-L, McCombe Waller S, Rogers MW. Lateral Perturbation-Induced Stepping: Strategies and Predictors in Persons Poststroke. J Neurol Phys Ther. 2017;41(4):222-228. doi:10.1097/NPT.0000000000000202
- Holloway RG, Tuttle D, Baird T, Skelton WK. The safety of hospital stroke care. Neurology. 2007;68(8):550-555. doi:10.1212/01.wnl.0000254992.39919.2e
- Andersson AG, Kamwendo K, Seiger A, Appelros P. How to identify potential fallers in a stroke unit: validity indexes of 4 test methods. J Rehabil Med. 2006;38(3):186-191. doi:10.1080/16501970500478023
- Belgen B, Beninato M, Sullivan PE, Narielwalla K. The association of balance capacity and falls self-efficacy with history of falling in community-dwelling people with chronic stroke. Arch Phys Med Rehabil. 2006;87(4):554-561. doi:10.1016/j.apmr.2005.12.027
- Mackintosh SFH, Hill K, Dodd KJ, Goldie P, Culham E. Falls and injury prevention should be part of every stroke rehabilitation plan. Clin Rehabil. 2005;19(4):441-451. doi:10.1191/0269215505cr796oa
- Mackintosh SF, Hill KD, Dodd KJ, Goldie PA, Culham EG. Balance score and a history of falls in hospital predict recurrent falls in the 6 months following stroke rehabilitation. Arch Phys Med Rehabil. 2006;87(12):1583-1589. doi:10.1016/j.apmr.2006.09.004
- Soyuer F, Oztürk A. The effect of spasticity, sense and walking aids in falls of people after chronic stroke. Disabil Rehabil. 2007;29(9):679-687. doi:10.1080/09638280600925860
- Lamb SE, Ferrucci L, Volapto S, Fried LP, Guralnik JM, Women’s Health and Aging Study. Risk factors for falling in home-dwelling older women with stroke: the Women’s Health and Aging Study. Stroke. 2003;34(2):494-501.
- Harris JE, Eng JJ, Marigold DS, Tokuno CD, Louis CL. Relationship of balance and mobility to fall incidence in people with chronic stroke. Phys Ther. 2005;85(2):150-158. doi:10.1093/ptj/85.2.150
- Hyndman D, Ashburn A. Stops walking when talking as a predictor of falls in people with stroke living in the community. J Neurol Neurosurg Psychiatry. 2004;75(7):994-997. doi:10.1136/jnnp.2003.016014
- Hyndman D, Ashburn A. People with stroke living in the community: Attention deficits, balance, ADL ability and falls. Disabil Rehabil. 2003;25(15):817-822. doi:10.1080/0963828031000122221
- Watanabe Y. Fear of falling among stroke survivors after discharge from inpatient rehabilitation. Int J Rehabil Res. 2005;28(2):149-152.
- Ramnemark A, Nilsson M, Borssén B, Gustafson Y. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke. 2000;31(7):1572-1577. doi:10.1161/01.str.31.7.1572
- Jørgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke. 2002;33(2):542-547. doi:10.1161/hs0202.102375
- Xu T, Clemson L, O’Loughlin K, Lannin NA, Dean C, Koh G. Risk Factors for Falls in Community Stroke Survivors: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2018;99(3):563-573.e5. doi:10.1016/j.apmr.2017.06.032
- Vojtikiv-Samoilovska D, Arsovska A. Prevalence and Predictors of Depression after Stroke – Results from a Prospective Study. Open Access Maced J Med Sci. 2018;6(5):824-828. doi:10.3889/oamjms.2018.182
- De Ryck A, Brouns R, Fransen E, et al. A prospective study on the prevalence and risk factors of poststroke depression. Cerebrovasc Dis Extra. 2013;3(1):1-13. doi:10.1159/000345557
- Nyberg L, Gustafson Y. Patient falls in stroke rehabilitation. A challenge to rehabilitation strategies. Stroke. 1995;26(5):838-842. doi:10.1161/01.str.26.5.838
- Suzuki T, Sonoda S, Misawa K, Saitoh E, Shimizu Y, Kotake T. Incidence and consequence of falls in inpatient rehabilitation of stroke patients. Exp Aging Res. 2005;31(4):457-469. doi:10.1080/03610730500206881
- Batchelor FA, Mackintosh SF, Said CM, Hill KD. Falls after stroke. Int J Stroke. 2012;7(6):482-490. doi:10.1111/j.1747-4949.2012.00796.x
- Gray VL, Juren LM, Ivanova TD, Garland SJ. Retraining postural responses with exercises emphasizing speed poststroke. Phys Ther. 2012;92(7):924-934. doi:10.2522/ptj.20110432
- Garland SJ, Stevenson TJ, Ivanova T. Postural responses to unilateral arm perturbation in young, elderly, and hemiplegic subjects. Arch Phys Med Rehabil. 1997;78(10):1072-1077. doi:10.1016/s0003-9993(97)90130-1
- Mansfield A, Wong JS, McIlroy WE, et al. Do measures of reactive balance control predict falls in people with stroke returning to the community? Physiotherapy. 2015;101(4):373-380. doi:10.1016/j.physio.2015.01.009
- Pigman J, Reisman DS, Pohlig RT, Wright TR, Crenshaw JR. The development and feasibility of treadmill-induced fall recovery training applied to individuals with chronic stroke. BMC Neurol. 2019;19(1):102. doi:10.1186/s12883-019-1320-8
- de Kam D, Geurts AC, Weerdesteyn V, Torres-Oviedo G. Direction-Specific Instability Poststroke Is Associated With Deficient Motor Modules for Balance Control. Neurorehabil Neural Repair. 2018;32(6-7):655-666. doi:10.1177/1545968318783884
- Mansfield A, Inness EL, Wong JS, Fraser JE, McIlroy WE. Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil Neural Repair. 2013;27(6):526-533. doi:10.1177/1545968313478486
- van Swigchem R, Roerdink M, Weerdesteyn V, Geurts AC, Daffertshofer A. The capacity to restore steady gait after a step modification is reduced in people with poststroke foot drop using an ankle-foot orthosis. Phys Ther. 2014;94(5):654-663. doi:10.2522/ptj.20130108
- Schinkel-Ivy A, Aqui A, Danells CJ, Mansfield A. Characterization of reactions to laterally directed perturbations in people with chronic stroke. Phys Ther. 2018;98(7):585-594. doi:10.1093/ptj/pzy039
- Wan J-J, Qin Z, Wang P-Y, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017;49(10):e384. doi:10.1038/emm.2017.194
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725-1789. doi:10.1152/physrev.2001.81.4.1725
- Kirkendall DT. Mechanisms of peripheral fatigue. Med Sci Sports Exerc. 1990;22(4):444-449.
- Knorr S, Ivanova TD, Doherty TJ, Campbell JA, Garland SJ. The origins of neuromuscular fatigue post-stroke. Exp Brain Res. 2011;214(2):303-315. doi:10.1007/s00221-011-2826-5
- Boudarham J, Roche N, Pradon D, Bonnyaud C, Bensmail D, Zory R. Variations in kinematics during clinical gait analysis in stroke patients. PLoS One. 2013;8(6):e66421. doi:10.1371/journal.pone.0066421
- Egerton T, Hokstad A, Askim T, Bernhardt J, Indredavik B. Prevalence of fatigue in patients 3 months after stroke and association with early motor activity: a prospective study comparing stroke patients with a matched general population cohort. BMC Neurol. 2015;15:181. doi:10.1186/s12883-015-0438-6
- Wong JS, Brooks D, Inness EL, Mansfield A. The Impact of Falls on Motor and Cognitive Recovery after Discharge from In-Patient Stroke Rehabilitation. J Stroke Cerebrovasc Dis. 2016;25(7):1613-1621. doi:10.1016/j.jstrokecerebrovasdis.2016.03.017
- Iosa M, Morone G, Fusco A, et al. Effects of walking endurance reduction on gait stability in patients with stroke. Stroke Res Treat. 2012;2012:810415. doi:10.1155/2012/810415
- Awad L, Reisman D, Binder-Macleod S. Distance-Induced Changes in Walking Speed After Stroke: Relationship to Community Walking Activity. J Neurol Phys Ther. 2019;43(4):220-223. doi:10.1097/NPT.0000000000000293
- Sibley KM, Tang A, Brooks D, McIlroy WE. Effects of extended effortful activity on spatio-temporal parameters of gait in individuals with stroke. Gait Posture. 2008;27(3):387-392. doi:10.1016/j.gaitpost.2007.05.007
- Sibley KM, Tang A, Patterson KK, Brooks D, McIlroy WE. Changes in spatiotemporal gait variables over time during a test of functional capacity after stroke. J Neuroeng Rehabil. 2009;6:27. doi:10.1186/1743-0003-6-27
- Park SW, Son SM, Lee NK. Exercise-induced muscle fatigue in the unaffected knee joint and its influence on postural control and lower limb kinematics in stroke patients. Neural Regen Res. 2017;12(5):765-769. doi:10.4103/1673-5374.206647
- Lewek MD, Bradley CE, Wutzke CJ, Zinder SM. The relationship between spatiotemporal gait asymmetry and balance in individuals with chronic stroke. J Appl Biomech. 2014;30(1):31-36. doi:10.1123/jab.2012-0208
- Rybar MM, Walker ER, Kuhnen HR, et al. The stroke-related effects of hip flexion fatigue on over ground walking. Gait Posture. 2014;39(4):1103-1108. doi:10.1016/j.gaitpost.2014.01.012
- Hyngstrom AS, Onushko T, Heitz RP, Rutkowski A, Hunter SK, Schmit BD. Stroke-related changes in neuromuscular fatigue of the hip flexors and functional implications. Am J Phys Med Rehabil. 2012;91(1):33-42. doi:10.1097/PHM.0b013e31823caac0
- Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22(1):51-56. doi:10.1016/j.gaitpost.2004.06.009
- Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait Posture. 1996;4(2):136-148. doi:10.1016/0966-6362(96)01063-6
- de Haart M, Geurts AC, Huidekoper SC, Fasotti L, van Limbeek J. Recovery of standing balance in postacute stroke patients: a rehabilitation cohort study. Arch Phys Med Rehabil. 2004;85(6):886-895. doi:10.1016/j.apmr.2003.05.012
- Dickstein R, Abulaffio N. Postural sway of the affected and nonaffected pelvis and leg in stance of hemiparetic patients. Arch Phys Med Rehabil. 2000;81(3):364-367. doi:10.1016/s0003-9993(00)90085-6
- Laufer Y, Sivan D, Schwarzmann R, Sprecher E. Standing balance and functional recovery of patients with right and left hemiparesis in the early stages of rehabilitation. Neurorehabil Neural Repair. 2003;17(4):207-213. doi:10.1177/0888439003259169
- Bensoussan L, Viton J-M, Schieppati M, et al. Changes in postural control in hemiplegic patients after stroke performing a dual task. Arch Phys Med Rehabil. 2007;88(8):1009-1015. doi:10.1016/j.apmr.2007.05.009
- Nardone A, Galante M, Lucas B, Schieppati M. Stance control is not affected by paresis and reflex hyperexcitability: the case of spastic patients. J Neurol Neurosurg Psychiatry. 2001;70(5):635-643. doi:10.1136/jnnp.70.5.635
- Marigold DS, Eng JJ. The relationship of asymmetric weight-bearing with postural sway and visual reliance in stroke. Gait Posture. 2006;23(2):249-255. doi:10.1016/j.gaitpost.2005.03.001
- Peurala SH, Könönen P, Pitkänen K, Sivenius J, Tarkka IM. Postural instability in patients with chronic stroke. Restor Neurol Neurosci. 2007;25(2):101-108.
- Mizrahi J, Solzi P, Ring H, Nisell R. Postural stability in stroke patients: vectorial expression of asymmetry, sway activity and relative sequence of reactive forces. Med Biol Eng Comput. 1989;27(2):181-190. doi:10.1007/bf02446228
- Little VL, McGuirk TE, Patten C. Impaired limb shortening following stroke: what’s in a name? PLoS One. 2014;9(10):e110140. doi:10.1371/journal.pone.0110140
- Burpee JL, Lewek MD. Biomechanical gait characteristics of naturally occurring unsuccessful foot clearance during swing in individuals with chronic stroke. Clin Biomech (Bristol, Avon). 2015;30(10):1102-1107. doi:10.1016/j.clinbiomech.2015.08.018
- Zhang F, Bohlen P, Lewek MD, Huang H. Prediction of intrinsically caused tripping events in individuals with stroke. IEEE Trans Neural Syst Rehabil Eng. 2017;25(8):1202-1210. doi:10.1109/TNSRE.2016.2614521
- Cruz TH, Dhaher YY. Impact of ankle-foot-orthosis on frontal plane behaviors post-stroke. Gait Posture. 2009;30(3):312-316. doi:10.1016/j.gaitpost.2009.05.018
- Lamontagne A, Malouin F, Richards CL. Locomotor-specific measure of spasticity of plantarflexor muscles after stroke. Arch Phys Med Rehabil. 2001;82(12):1696-1704. doi:10.1053/apmr.2001.26810
- Burridge JH, Wood DE, Taylor PN, McLellan DL. Indices to describe different muscle activation patterns, identified during treadmill walking, in people with spastic drop-foot. Med Eng Phys. 2001;23(6):427-434. doi:10.1016/s1350-4533(01)00061-3
- Cruz TH, Lewek MD, Dhaher YY. Biomechanical impairments and gait adaptations post-stroke: multi-factorial associations. J Biomech. 2009;42(11):1673-1677. doi:10.1016/j.jbiomech.2009.04.015
- Lewek MD, Hornby TG, Dhaher YY, Schmit BD. Prolonged quadriceps activity following imposed hip extension: a neurophysiological mechanism for stiff-knee gait? J Neurophysiol. 2007;98(6):3153-3162. doi:10.1152/jn.00726.2007
- Darekar A, Lamontagne A, Fung J. Locomotor circumvention strategies are altered by stroke: I. Obstacle clearance. J Neuroeng Rehabil. 2017;14(1):56. doi:10.1186/s12984-017-0264-8
- Den Otter AR, Geurts ACH, de Haart M, Mulder T, Duysens J. Step characteristics during obstacle avoidance in hemiplegic stroke. Exp Brain Res. 2005;161(2):180-192. doi:10.1007/s00221-004-2057-0
- Ma C, Chen N, Mao Y, Huang D, Song R, Li L. Alterations of Muscle Activation Pattern in Stroke Survivors during Obstacle Crossing. Front Neurol. 2017;8:70. doi:10.3389/fneur.2017.00070
- Lamontagne A, Richards CL, Malouin F. Coactivation during gait as an adaptive behavior after stroke. J Electromyogr Kinesiol. 2000;10(6):407-415.
- Kitatani R, Ohata K, Sakuma K, et al. Ankle muscle coactivation during gait is decreased immediately after anterior weight shift practice in adults after stroke. Gait Posture. 2016;45:35-40. doi:10.1016/j.gaitpost.2016.01.006
- Intiso D, Santilli V, Grasso MG, Rossi R, Caruso I. Rehabilitation of walking with electromyographic biofeedback in foot-drop after stroke. Stroke. 1994;25(6):1189-1192. doi:10.1161/01.str.25.6.1189
- Cozean CD, Pease WS, Hubbell SL. Biofeedback and functional electric stimulation in stroke rehabilitation. Arch Phys Med Rehabil. 1988;69(6):401-405.
- Drużbicki M, Guzik A, Przysada G, Kwolek A, Brzozowska-Magoń A. Efficacy of gait training using a treadmill with and without visual biofeedback in patients after stroke: A randomized study. J Rehabil Med. 2015;47(5):419-425. doi:10.2340/16501977-1949
- Nott CR, Neptune RR, Kautz SA. Relationships between frontal-plane angular momentum and clinical balance measures during post-stroke hemiparetic walking. Gait Posture. 2014;39(1):129-134. doi:10.1016/j.gaitpost.2013.06.008
- Bhatt T, Dusane S, Patel P. Does severity of motor impairment affect reactive adaptation and fall-risk in chronic stroke survivors? J Neuroeng Rehabil. 2019;16(1):43. doi:10.1186/s12984-019-0510-3
Evaluation
We received constant feedback from Dr. Lewek as we researched for and wrote this review paper. We were able to meet with him and incorporate his feedback weekly. After the final draft had been completed, we sent it to our committee members, Dr. Mercer and Dr. Hamilton, and received additional feedback on this initial draft. These committee members also completed the following Evaluation Form to assess the quality of our review paper and its ability to communicate the desired information. We were able to incorporate their feedback into this final draft. (See Evaluation Form_Hamilton and Evaluation Form _Mercer for their respective completed feedback forms.) We plan to incorporate further edits from our peers and other researchers into this paper with the hope of publication.

2 Responses to “Understanding the Multifactorial Causes of Falls in Individuals Post-Stroke”
Debbie Thorpe
Kristen
Wow! You and Kristen did a fantastic job on this project. Congratulations on your poster abstract acceptance for HMSC Day. Hopefully, you can submit to a state or national conference in the future! The manuscript is well written and very informative. I can’t wait to see it in publication! You all worked as a very productive team!
Best Wishes….
Vicki Mercer
Nice work on this project! Glad to see that you are planning to pursue publication. Stick with that effort – persistence usually pays off! Best wishes to you both-