Created by: Amanda Doty
During my time in PT school, I developed an interest in working with individuals with neurological conditions. Beginning in the fall (2016) semester, I had the opportunity to participate in a research experience with Dr. Michael Lewek, PT, PhD, working with patients post-stroke. The purpose of our study is to determine whether individuals with chronic hemiparesis following a stroke have the capacity to learn to increase their paretic limb propulsion when a posteriorly directed resistance force is applied to the body’s COM during a single-session of treadmill walking. Our study included data collection and analysis from nine unimpaired individuals and six individuals with chronic stroke. I completed a Critically Appraised Topic (CAT) on the effects of COM-specific interventions on paretic limb propulsion during the fall semester. In the spring semester, I submitted an abstract, which was selected for poster presentation at the UNC Human Movement Science Research symposium on March 31, 2017. I received valuable feedback at the symposium from fellow researchers, which has helped me assess the quality of this study, better understand how to interpret the results, and determine the clinical relevance. I am currently working on a manuscript of the findings from this study with the guidance of Dr. Lewek.
Acknowledgements
I would like to acknowledge my advisor, Dr. Michael Lewek, PT, PhD who offered his guidance and expertise for this project. I would also like to acknowledge and thank my fellow classmate and research partner, Cristina Raiti, SPT, who also participated in this research study. Finally, I would like to acknowledge my committee members, Vicki Mercer, PT, PhD, and Catherine Jacobs, DPT, who provided valuable feedback on my capstone materials. Posted below is a copy of the abstract and poster presentation that I presented at the Human Movement Science Research Symposium. Following that is an overview of the background and methods sections of my manuscript.
ABSTRACT
Introduction: Individuals with hemiparesis post-stroke commonly demonstrate gait abnormalities that lead to difficulty with community ambulation and an increased risk of falls. These gait deficits have been strongly associated with reduced paretic limb propulsion despite intensive rehabilitation. Reduced limb propulsion may be due to inadequate muscle strength or an inability to access available strength. Because evidence suggests that additional force generating capacity is available, we believe that we can use motor adaptation to coax additional force from the limb during gait.
Objective: The purpose of this study was to determine whether individuals with chronic hemiparesis following a stroke have the capacity to learn to increase their paretic limb propulsion when a posteriorly directed resistance force is applied to the body’s COM during a single-session of treadmill walking.
Methods: Six participants with chronic stroke (>6 months post-stroke) and 9 unimpaired subjects participated in this study. The first two minutes (Baseline) and the last two minutes (post-adaptation) consisted of unperturbed walking. An applied posterior resistance force of ~5-7.5% of subject’s body weight was applied to the pelvis (i.e. COM) for the middle ten minutes (adaptation). Participants wore an overhead harness for safety and were allowed to use the handrail for balance only. Kinematic and kinetic data were collected continuously to determine each steps peak limb propulsion, peak plantar flexion moment, peak propulsive impulse, and the trailing limb angle (TLA). Given the small sample size and preliminary nature of the project, data analysis is qualitative in nature.
Results: The stroke participants showed a gradual increase in their paretic propulsive limb forces with the addition of a posterior resistance force to the COM. Two of the participants appeared to increase their propulsive force through greater plantar flexion moment while the others used a different strategy. No robust aftereffect was observed and propulsive force returned to baseline once the resistance force was removed. The unimpaired participants showed an immediate increase in peak propulsive force with the addition of a posterior resistance force to their COM that was maintained throughout the 10 minutes. Their propulsive force also returned to baseline after the removal of the resistance force.
Conclusion: Individuals with chronic hemiparesis following stroke have the capacity to increase their paretic propulsive limb forces when a posterior resistance force is applied to their COM during gait. A differential response was observed; some individuals increased their propulsive force through greater plantarflexion moment, while others used another strategy, and certain individuals showed a greater response than others. The absence of an aftereffect suggests that there is a lack of feed-forward mechanism.
(Click to enlarge)
BACKGROUND
Though the rate of death due to stroke has declined over the past 10 years in the United States, stroke remains the leading cause of long-term disability.1 Walking is often reported to be the most important goal for rehabilitation by individuals post-stroke.2 Individuals with stroke commonly demonstrate gait abnormalities3 that lead to difficulty with community ambulation and an increased risk of falls.4 Impairment in the paretic leg continue into the chronic stage of stroke and often results in slow gait speed.5,6 Gait speed is often used to assess walking ability for community ambulation, which is why increasing gait speed is commonly the focus of gait rehabilitation. Despite intensive rehabilitation, deficits in gait speed persist.7
Individuals with post-stroke hemiparesis demonstrate decreased propulsive force of the paretic limb,8 defined as the anterior component of the ground reaction force. The reduction in paretic propulsion is a major contributor to reduced gait speed and overall walking ability.3,5 The decrease in paretic propulsion may be due to weakness of the plantar flexor muscles,9 given their contribution to propulsive force in the late stance phase of unimpaired gait.10,11 Recently, post-stroke gait rehabilitation has focused on targeting the propulsive force of the paretic limb.12–14 Hurt et al found that individuals post-stroke have the ability to generate higher propulsive forces than what they typically use.15
Understanding the mechanisms that could lead to increased propulsive limb force would help determine the best strategies for use in post-stroke rehabilitation. In particular, the limb’s propulsive force is highly dependent on both the ankle plantar flexion moment and the trailing limb angle (TLA).14,16,17 Both the TLA and the plantar flexion moment contribute linearly to propulsive force, with the TLA contributing almost twice as much as the ankle moment in unimpaired individuals.18 These findings suggest that interventions directed at either increasing the TLA or ankle moment may be effective for increasing propulsive force.17
Previous work has specifically targeted the plantarflexion moment and the trailing limb angle to attempt to increase propulsive limb force. For example, functional electrical stimulation (FES) to the plantar flexor muscles coupled with fast walking speed (to increase TLA) during treadmill walking,18–20 successfully enabled the production of greater propulsive force in the paretic limb.20 The addition of FES to the ankle plantar flexors at terminal stance appears critical to provide greater forward propulsion, as stimulation to the dorsiflexors during the swing phase, as commonly used for ‘foot drop’, was unable to increase propulsion.21 Hsiao et al determined that the TLA contributed the most to increases in propulsive force and only found an increase in plantar flexion moment when functional electrical stimulation was added to individuals who performed fast-pace walking.14 Specific interventions directed at increasing the TLA or the paretic ankle moment, such as functional electrical stimulation or directly strengthening the ankle plantar flexors, may be necessary to increase propulsive limb force.14
Individuals post-stroke have the ability to adapt their movement pattern to an altered environment.22–25 Using techniques of motor adaptation, the neuromechanical capacity to learn different movements can be revealed. In particular, improvements in step length symmetry have been shown with repeated application of walking on a split-belt treadmill,25 or with robotic resistance to swing.22 The use of motor adaptation provides a window into the ability to learn or produce different movement patterns. These are revealed as aftereffects. Creation of aftereffects requires the use of error-based training. In the case of reduced paretic propulsion, an increase in error would be to pull backwards on the participant, such that the participant needs to provide greater propulsive force to overcome the induced error. Although motor adaptation of certain movements is possible post-stroke, what remains unknown is how individuals post-stroke will adapt to the need to increase propulsive force. Altering the environment to require greater propulsive force will provide great insight into the ability of individuals post-stroke to learn to increase propulsive limb forces.
Previous studies suggest that individuals with chronic stroke have the ability to increase their paretic propulsive limb force and increase their gait speed through the use of other interventions.14,15,22,23 The purpose of this study was to determine the effects of a posteriorly directed resistance force to the body’s COM on paretic limb propulsion in patients with hemiparesis post-stroke, specifically after the resistance force is removed. We hypothesized that: when the resistance force is first applied, the stroke participants would demonstrate decreased paretic propulsive forces due to the posterior resistance force. We also hypothesized that, following removal of the resistance, individuals with hemiparesis post-stroke would have adapted to generate increased propulsive force in the paretic limb. This hypothesis is based on previous findings that suggest a swing resistance force may result in an aftereffect of improved step length symmetry,23 step cadence, and single-leg support time of the paretic leg.22 The outcomes of this study are expected to help guide clinical practice by determining potential intervention strategies to incorporate into gait training for individuals post-stroke.
METHODS
Participants
Six individuals with chronic (>6 months) stroke (4 M/2 F; age: 56 + 15) and nine unimpaired individuals (5 M/4 F; age: 29 + 6.3) were included in the study. Stroke participants presented with lower extremity hemiparesis (4R/2L) resulting from ischemic or hemorrhagic unilateral brain lesion, and one stroke participant had an aneurysm. All unimpaired subjects had their right side tested. Subjects were excluded from this study if they could not walk without physical assistance, required an ankle foot orthosis (AFO) for ambulation, had any self-reported pre-existing cardiovascular, metabolic, or musculoskeletal condition(s) that prevented treadmill walking, or a separate neurological condition that could also affect their walking ability. All participants signed an informed consent form approved by the IRB of the University of North Carolina at Chapel Hill before participating.
Protocol
All testing occurred in a single-session and consisted of 14 minutes of walking performed on a dual-belt instrumented treadmill. All participants wore an overhead safety harness that did not restrict lower extremity movement or provide body weight support. Prior to treadmill walking, each stroke participant was assessed using the LE Fugl-Meyer to characterize sensorimotor control of the lower extremities. They then performed two passes of over ground walking across a 10ft Zeno Walkway (ProtoKinetics, Havertown, PA) to determine comfortable walking speed and 2 passes to determine fast walking speed. The treadmill speed was then set to the participant’s measured comfortable walking speed, or slightly slower than their measured comfortable walking speed. Treadmill speeds were often chosen slower to allow for participants to be able to walk for up to 14 minutes without stopping and to be able to manage the prolonged walking with a posterior restraining force. Participants were permitted to use the handrails as needed for balance but were encouraged not to use it to support body weight.
 We produced a posterior restraining force, applied to the participants’ center of mass (COM) as they walked on the treadmill. Participant’s walked for the first 2 minutes on the treadmill at a constant speed without any resistance. Next, without stopping the treadmill, a resistance of ~5-7.5% of their body weight was applied to the COM as they continued to walk at a constant speed for 10 minutes. The force was applied by stretched theratubing that was monitored in real time using a tension/compression load cell (MLP 150, Transducer Techniques, Temecula, CA). For the final 2 minutes, the resistive force was quickly removed and the participants walked without any resistance. Participants were alerted prior to the application and removal of the resistance force during testing.
We produced a posterior restraining force, applied to the participants’ center of mass (COM) as they walked on the treadmill. Participant’s walked for the first 2 minutes on the treadmill at a constant speed without any resistance. Next, without stopping the treadmill, a resistance of ~5-7.5% of their body weight was applied to the COM as they continued to walk at a constant speed for 10 minutes. The force was applied by stretched theratubing that was monitored in real time using a tension/compression load cell (MLP 150, Transducer Techniques, Temecula, CA). For the final 2 minutes, the resistive force was quickly removed and the participants walked without any resistance. Participants were alerted prior to the application and removal of the resistance force during testing.
During walking, lower extremity and pelvic motion were recorded using an 8-camera motion capture system (Vicon, Denver, CO) sampling at 120 Hz. Retro-reflective markers were attached to the participants’ pelvis, legs, and feet to track movement as described previously by Murray et al.26 All joint markers were in place prior to testing for a static standing calibration. The ground reaction force (GRF) was measured from the treadmill (Bertec, Worthington, OH) and sampled concurrently with the posterior resistance force at 1200 Hz
Data Management and Processing
The marker trajectories and ground reaction force data were filtered with a recursive 6 Hz and 20 Hz low pass filter, respectively. From these filtered data, the peak propulsive force was measured on a step-by-step basis as the peak of the anteriorly directed GRF. The peak plantar flexion moment was computed using inverse dynamics using Visual 3D (C-motion, Bethesda, MD), and the TLA was computed as the angle between a vertical line from the pelvis’s COM and a vector from the COM of the pelvis to the center of pressure (COP) from the foot. Data were analyzed from specific timeframes including: Baseline (minute1-2), Adaptation (minutes 3-12), and Post-adaptation (minutes 13-14). Specifically, we were interested in the transition between these conditions. Thus we analyzed the final five strides from the end of minute 2 and 12, as well as the initial five strides from the beginning of minute 3, 13, and 14.
Statistical Analysis
Outcome measures were analyzed with SPSS (version 24). Three repeated measures ANCOVA’s (repeated for time and controlled for gait speed) were performed to assess group, time, and interaction effects for the peak propulsive peak, the peak plantarflexion moment, and the TLA. Bonferroni corrected t-tests were also used.
RESULTS
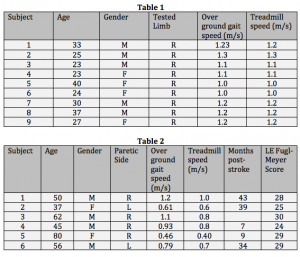
We are continuing to collect and analyze data for this study, so the results are not complete at this time. Demographics for the 9 unimpaired participants are described in Table 1 below. Demographics for the 6 stroke participants are described in Table 2 below.
References:
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation. 2016;133(4):e38-e48. doi:10.1161/CIR.0000000000000350.
- Bohannon RW, Andrews a. W, Smith MB. Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res. 1988;11(August):181-184. doi:10.1097/00004356-198806000-00012.
- Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. doi:10.1016/j.gaitpost.2004.06.009.
- Weerdesteyn V, de Niet M, van Duijnhoven HJR, Geurts ACH. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195-1213. doi:10.1682/JRRD.2007.09.0145.
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37(3):872-876. doi:10.1161/01.STR.0000204063.75779.8d.
- Alabdulwahab SS, Ahmad F, Singh H. Effects of Functional Limb Overloading on Symmetrical Weight Bearing, Walking Speed, Perceived Mobility, and Community Participation among Patients with Chronic Stroke. Rehabil Res Pract. 2015;2015:241519. doi:10.1155/2015/241519.
- Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke. 2010;41(1):129-135. doi:10.1161/STROKEAHA.109.563247.
- Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22(1):51-56. doi:10.1016/j.gaitpost.2004.06.009.
- Turns LJ, Neptune RR, Kautz SA. Relationships Between Muscle Activity and Anteroposterior Ground Reaction Forces in Hemiparetic Walking. doi:10.1016/j.apmr.2007.05.027.
- Liu MQ, Anderson FC, Pandy MG, Delp SL. Muscles that support the body also modulate forward progression during walking. J Biomech. 2006;39:2623-2630. doi:10.1016/j.jbiomech.2005.08.017.
- Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech. 1999;14:125-135.
- Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA. Targeting paretic propulsion to improve poststroke walking function: a preliminary study. Arch Phys Med Rehabil. 2014;95(5):840-848. doi:10.1016/j.apmr.2013.12.012.
- Awad LN, Binder-Macleod SA, Pohlig RT, Reisman DS. Paretic Propulsion and Trailing Limb Angle Are Key Determinants of Long-Distance Walking Function After Stroke. Neurorehabil Neural Repair. 2015;29(6):499-508. doi:10.1177/1545968314554625.
- Hsiao HY, Knarr BA, Pohlig RT, Higginson JS, Binder-Macleod SA. Mechanisms used to increase peak propulsive force following 12-weeks of gait training in individuals poststroke. J Biomech. 2016;49(3):388-395. doi:10.1016/j.jbiomech.2015.12.040.
- Tyrell CM, Roos MA, Rudolph KS, Reisman DS. Influence of Systematic Increases in Treadmill Walking Speed on Gait Kinematics After Stroke. Phys Ther. 2011;91(3):392-403. doi:10.2522/ptj.20090425.
- Hsiao HY, Knarr BA, Higginson JS, Binder-Macleod SA. The relative contribution of ankle moment and trailing limb angle to propulsive force during gait. Hum Mov Sci. 2015;39:212-221. doi:10.1016/j.humov.2014.11.008.
- Hsiao H, Knarr BA, Higginson JS, Binder-Macleod SA. Mechanisms to increase propulsive force for individuals poststroke. J Neuroeng Rehabil. 2015;12:40. doi:10.1186/s12984-015-0030-8.
- Hurt CP, Wang J, Capo-Lugo CE, Brown DA. Effect of progressive horizontal resistive force on the comfortable walking speed of individuals post-stroke. J Neuroeng Rehabil. 2015;12:12. doi:10.1186/s12984-015-0007-7.
- Kesar TM, Perumal R, Reisman DS, et al. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: effects on poststroke gait. Stroke. 2009;40(12):3821-3827. doi:10.1161/STROKEAHA.109.560375.
- Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA. Targeting Paretic Propulsion to Improve Poststroke Walking Function: A Preliminary Study. Arch Phys Med Rehabil. 2014;95:840-848. doi:10.1016/j.apmr.2013.12.012.
- Kesar TM, Perumal R, Reisman DS, et al. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: Effects on poststroke gait. Stroke. 2009;40(12):3821-3827. doi:10.1161/STROKEAHA.109.560375.
- Wu M, Landry JM, Kim J, Schmit BD, Yen S-C, Macdonald J. Robotic resistance/assistance training improves locomotor function in individuals poststroke: a randomized controlled study. Arch Phys Med Rehabil. 2014;95(5):799-806. doi:10.1016/j.apmr.2013.12.021.
- Yen S-C, Schmit BD, Wu M. Using swing resistance and assistance to improve gait symmetry in individuals post-stroke. Hum Mov Sci. 2015;42:212-224. doi:10.1016/j.humov.2015.05.010.
- Savin DN, Morton SM, Whitall J. Generalization of improved step length symmetry from treadmill to overground walking in persons with stroke and hemiparesis. Clin Neurophysiol. 2014;125(5):1012-1020. doi:10.1016/j.clinph.2013.10.044.
- Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair. 2013;27(5):460-468. doi:10.1177/1545968312474118.
- Murray M, Hardee A, Goldberg RL, Lewek MD. Loading and knee flexion after stroke: Less does not equal more. J Electromyogr Kinesiol. 2014;24(1):172-177. doi:10.1016/j.jelekin.2013.10.006.
*Image obtained from: https://news.uthscsa.edu/salsi-awards-200000-brain-health-research-initiatives/


4 Responses to “LEARNING TO BE FORCEFUL: MOTOR ADAPTATION FOR INCREASING PARETIC PROPULSION POST-STROKE”
Amanda Doty
Thanks Mike! I learned so much from this experience and I truly appreciate all of your guidance throughout the process. This project has only reinforced my desire to work with this patient population. Thank you again for all of the time you spent instructing and leading Cristina and I through the research process. I look forward to continuing with our data analysis and completing the manuscript!
Mike Lewek
Amanda
Nice job on this project. It was a pleasure to work with both you and Cristina this year. You took a technically challenging project and were able to independently collect data from multiple sources. These data are valuable for showing the learning capabilities of individuals post-stroke. Well done (I’m copy/pasting the same comments to Cristina since you worked so well together).
Mike
Amanda Doty
Hi Vicki,
Thank you for your feedback! Yes, the large age gap between the unimpaired and stroke participant is certainly a limitation in our study. We had some trouble with recruitment, but this is definitely something to think about in the future, and I will make sure and include this in the manuscript.
I participated in all of the data collection for this study. Mike Lewek taught Cristina and me how to use all of the equipment in the lab including the Zeno Mat, the dual belt treadmill, cameras, etc. We performed the testing for all of the unimpaired participants on our own, and Mike was present for testing with all of the stroke participants. Cristina and I calibrated the cameras, placed the reflective markers, and operated the computer and treadmill during testing. Cristina manually held the theratubing and added the resistance force during testing. This was a very valuable learning experience for me! One “lesson learned” was to take our time with set-up because it helped our data analysis later on. We also learned how to problem solve when there were technical difficulties. We are still working on our data analysis, but hope to finish the manuscript very soon. Thanks!
Vicki Mercer
Hi Amanda-
Very interesting project, with clear implications for clinical practice! I notice that there is a fairly large difference in age between subjects with stroke and the comparison group. Is there any concern that effects of stroke could be confounded with age-related changes in force generating capacity?
How much were you involved with data collection for all of the subjects? I’d love to hear a little more about your role, and any “lessons learned” from your involvement with this research.
Great work! Will look forward to seeing your name on the journal publication-
Vicki