Rhythmic Auditory Stimulation for Improved Gait in Parkinson’s Disease
By: Sarah Stevenson, SPT
Background
As I began to think about electives for my third and final year of physical therapy school, I realized I had never participated in research, even after attending a research based institution for my undergraduate studies. After looking over the research that our faculty was conducting, Dr. Mike Lewek’s project caught my eye. He was expanding a project that Maddie Wygand and Guneet Chawla had conducted the prior year, on the effects of auditory cues and gait training in patients with Parkinson’s disease. My grandfather had Parkinson’s disease and I remember learning about Parkinsonian gait in our first or second year and realizing that was exactly how my grandpa used to walk. When I saw that Dr. Lewek was conducting research to address these changes in gait, I decided I had to be part of the project. Ali Sherron and I were the co-investigators on this project, along with Dr. Lewek and Dr. Nina Browner, MD. Maddie and Guneet’s previous study found that by using a metronome for rhythmic auditory stimulation (RAS) and gait training on a treadmill, patients were able to change their step length. Ali, Dr. Lewek, Dr. Browner, and I decided to expand on this and see what happens to participants’ spatiotemporal measures of gait and balance after training with RAS on a treadmill and overground for six weeks, training three times each week.
During Evidence Based Practice II, I was able to delve into literature to see if there was any information on gait training with RAS in patients with Parkinson’s disease and the effects it has on balance. I found while creating my CAT that there was a lack of literature of the effects of this intervention on balance. This was further evidence that our study was needed to help identify if this intervention had any effects on balance, along with the gait changes.
After conducting our research study, Ali and I put together a poster which we presented at the 2019 Human Movement Science Research Symposium on campus. In preparation of creating the poster, Ali and I reviewed guidelines for how to format a poster for a presentation and also information for presenting the poster. Similar to my CAT, I focused on the balance effects of our intervention, while Ali examined the spatiotemporal measures of gait.
Acknowledgements
Dr. Mike Lewek, PT, PhD- Thank you for your support and guidance throughout this study and all the help you have provided over the past three years. You have answered our countless questions and provided us with valuable feedback throughout our research and with our manuscript. I am so appreciative for all you have done for us!
Dr. Nina Browner, MD- Thank you for your guidance with our research and for being a member of our Capstone Committee. Your assistance with finding participants for our research study is greatly appreciated as well as your added expertise with Parkinson’s disease.
Diane Meyer, PT, MSCS- Thank you for serving on our Capstone Committee and providing Ali and I with timely feedback on our manuscript. Your insight as a clinician provided us with very valuable feedback.
Ali Sherron, SPT- Thank you for all the hard work and support you’ve given me throughout this project, and also throughout the past three years of school. You always made our 6 a.m. training sessions entertaining and knew how to make sense of my thoughts and turn them into words to use in our manuscript. Thanks for everything!
Abstract
Introduction
Parkinson’s disease is often associated with deficits in gait speed and other spatiotemporal measures, which can negatively impact balance and community mobility. Rhythmic auditory stimulation has the potential to improve spatiotemporal measures of gait in this population through entertainment of movement patterns. Cadence and step length can be differentially affected on a treadmill and overground by strategically selecting frequencies appropriate to the parameter to cause even greater increases in gait speed. However, rhythmic auditory stimulation has shown limited ability to impact balance. Incorporating rhythmic auditory stimulation during treadmill training and overground walking has the ability to improve static and dynamic balance, as well as the ability to improve spatiotemporal measures of gait in community-dwelling older adults with Parkinson’s disease.
Purpose
The purpose of this study was to perform an exploratory analysis of the effect of training with rhythmic auditory stimulation on gait speed, cadence, stride length, and static and dynamic balance.
Methods
Three individuals participated in this exploratory pilot study. A 72-year-old man, a 66-year-old woman, and a 75-year-old female (all with Hoehn-Yahr Stage 2 Parkinson’s disease) participated in 6 weeks of gait training with rhythmic auditory stimulation to improve gait speed and spatiotemporal measures. Each session combined treadmill-based gait training followed by overground gait training. The rhythmic auditory stimulation was provided with a metronome set to 85% and 115% of their self-selected cadence for treadmill and overground training, respectively. We performed clinical and laboratory tests of gait and balance prior to training, following completion of training, and at a 3 month follow up.
Results
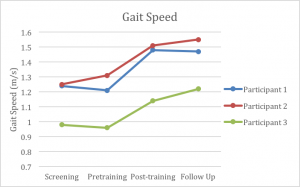
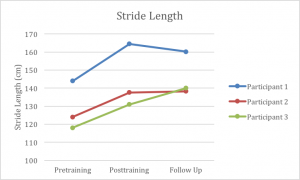
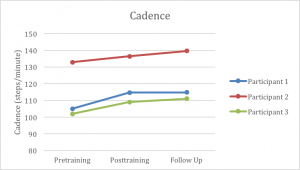
All three participants improved overground gait speed (participant 1: +0.27 m/s; participant 2: +0.20 m/s; participant 3: +0.18 m/s), cadence (6.76 ±3.07 steps per minute), and stride length (15.7 ±4.17 cm). There were relatively minor to no changes in balance for all participants.
Conclusion
All three participants made improvements in gait speed and spatiotemporal measures. Continued work is needed to further determine the impact rhythmic auditory stimulation has on balance in this population. Further research is also warranted for determination of long term improvements and the feasibility of use in the community setting.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that can cause impairments in motor and postural control, gait, automaticity of movement, and static and dynamic balance.1 Individuals with PD commonly present with distinct gait deficits such as bradykinesia, decreased arm swing, decreased stride length, and decreased cadence, resulting in shuffled steps.2,3 The lack of movement automaticity in individuals with PD can also contribute to freezing of gait, which further increases the risk of falls.4 Pharmacological management is often a major part of treatment for PD given its ability to increase dopamine stores and reduce disease symptoms; however, medications can be ineffective at improving gait deficits.5,6 Additionally, prolonged drug therapy may increase episodes of freezing of gait, thus increasing the risk of falls.7 In order to properly address the gait deficits for individuals with PD, intensive gait rehabilitation is warranted as an adjunct to drug therapy.7
The use of external cues represents a well-established technique that is commonly implemented during rehabilitation for individuals with PD.8 Individuals with PD have impaired internal pacing but are able to use external timing cues to improve the rhythmicity of gait.9–11 As an example, walking on a treadmill provides external somatosensory cues (i.e., speed of the treadmill belt) to drive the stepping pattern.12 Furthermore, treadmill walking appears to reduce the elevated prefrontal cortex activity for people with PD.13 This reduction in frontal lobe activation may contribute to long term improvements, by promoting greater gait automaticity.13
Auditory cues, such as musical beats, metronomes, and rhythmic clapping, represent another form of external cueing that is commonly implemented in conjunction with gait training for individuals with PD.3 Walking with auditory cues can have a positive impact on gait initiation, and reduce freezing episodes and falls for individuals with PD.11 More specifically, metronomes offer an effective, clinically feasible strategy to improve spatiotemporal measures of gait such as gait speed, stride length, and cadence.3 In the overground environment, higher (i.e., faster) frequencies are advocated to elicit increased gait speed.14 Recently, Chawla et al suggested that slower metronome frequencies, when employed during treadmill training, are more effective than faster metronome frequencies at increasing step lengths during treadmill walking for individuals with PD.15 During overground walking, however, faster tempo metronomes appear to consistently increase cadence and gait speed, but are inconsistent at altering step length.15 Therefore, we propose that coupling slow cadence cues on a treadmill (to enhance stride length), followed immediately by faster cadence cues overground (to enhance cadence and gait speed), will elicit positive spatiotemporal gait features specifically targeting the known impairments for individuals with PD.
Increasing stride length has the potential to increase the time spent in single limb stance. Such a change may affect balance, although there is limited evidence regarding the effects of gait training with auditory cueing on balance outcomes. It is possible, however, that gait training with auditory cueing can improve motor control and anticipatory postural control, leading to improved balance.16
The carryover from the treadmill environment to the overground environment is critical for correcting multiple aspects of disordered walking in individuals with Parkinson’s disease. Therefore, the purpose of this case series was to describe the use of a novel pairing of both big, slow movements (obtained with slow tempo rhythmic auditory stimulation (RAS) on a treadmill) followed by high-intensity rapid movements (obtained during fast tempo RAS during overground walking) during gait training for individuals with Parkinson’s disease. We believe that this novel pairing of training conditions will address both the affected spatial and temporal aspects of gait. Furthermore, we hypothesize that improving gait mechanics will improve overall balance in individuals with PD.
Case Description
Participant 1: History and Systems Review
The first participant was a 72-year-old man diagnosed with Parkinson’s disease (PD) by his neurologist 4 years before our evaluation. According to his neurologist, his PD stage was classified as Hoehn-Yahr Stage 2. Besides his initial diagnosis of Parkinson’s disease, his past medical history was unremarkable. During training he was taking both Levodopa 25 100mg and Pramipexole 1 mg three times per day. Prior to his diagnosis, he was very physically active and enjoyed going to the gym several times per week. After his diagnosis he continued going to the gym but noticed a decrease in his ability to ambulate safely on the treadmill and began using the stationary bike. Upon his initial evaluation with us, he expressed an interest to increase his walking speed in order to improve his ability to keep up with his grandchildren and reduce freezing episodes.
Clinical Impression and Examination
Due to his diagnosis of Parkinson’s disease, we were concerned about the possibility of decreased stride length, gait speed, and balance and the effects these could have on safety when ambulating in the community and his risk of falls. During his initial evaluation, he scored 27/28 on the Mini-BESTest, 7/24 on the Freezing of Gait Questionnaire, and a 29/30 on the Montreal Cognitive Assessment.17–19 He completed the Four Square Step Test in 11.87 seconds and had a total score of 25 (12 on Left and 13 on Right) on the Step Test.20,21 He performed a Six-Minute Walk Test (6MWT) along a 100 foot hallway with a GaitRite mat (CIR Systems, Franklin, NJ) placed in the middle of the hallway. While ambulating across the GaitRite mat, PKMAS software (Protokinetics, Havertown, PA) calculated his gait speed, cadence, and stride length for each pass to determine an average value for the test (Table 1). An initial screening for the 6MWT was performed prior to the pre-test, to ensure consistent performance in gait speed and distance walked. Determination of spatiotemporal gait measures using pressure mats is a valid measure for individuals with Parkinson’s disease.22 Observationally, he appeared to ambulate at a slightly slower than normal speed, without the use of an assistive device, with slightly decreased arm swing bilaterally and decreased stride length for his stature, when compared to a normal gait speed is 1.3 – 1.4 m/s and normal stride length is 140 – 160 cm.23,24 The decrease in stride length and gait speed were hypothesized to be due, in part, to the subtle deficits in balance. He appeared to be a good candidate for gait training with rhythmic auditory stimulation, given his high level of motivation, desire to improve his walking, and his self-reported progression of symptoms.
Participant 2: History and Systems Review
The second participant was a 66-year-old woman, who was diagnosed eight years ago with PD. At the initial evaluation she presented with Hoehn-Yahr Stage 2, according to her neurologist. Besides the diagnosis of PD, she also had an unremarkable past medical history. During training she was taking Levodopa 25 100 mg two times per day, Amantadine 100 mg three times per day, and Ropinirole XL 6 mg once per day. Prior to her diagnosis, she was very physically active participating in dance recitals and going for walks around her neighborhood. After her diagnosis, she continued to work as a dance teacher, and walked in her neighborhood with her husband frequently, but she reported a decrease in her rhythm and coordination during dancing. Her goals were to improve her coordination and rhythm when walking and dancing.
Clinical Impression and Examination
As with participant 1, we were concerned about the possibility of deficits in spatiotemporal measures of gait due to her Hoehn-Yahr stage and diagnosis of Parkinson’s disease. During her initial evaluation, she stated that she had no pain. She scored a 25/28 on the Mini-BESTest, a 3/24 on the Freezing of Gait Questionnaire, and a 30/30 on the Montreal Cognitive Assessment. She completed the Four Square Step Test in 6.35 seconds and had a total of 41 (21 on Left and 20 on Right) on the Step Test. She also performed the 6MWT while ambulating over the GaitRite mat to assess spatiotemporal aspects of her gait were assessed (Table 2). Again, her screening and pre-test results on the 6MWT were consistent. Visually she was observed to have decreased stride lengths and a quickened cadence. She ambulated without an assistive device but had a wide base of support. We attributed her decreased stride length and increased base of support to her reported feelings of being unstable. Given her findings, we believed that she had a good chance of improving her gait with gait training with rhythmic auditory stimulation.
Participant 3: History and Systems Review
The third participant was a 75-year-old woman diagnosed with Parkinson’s disease (Hoehn-Yahr Stage 2). She was initially diagnosed with PD one year prior to evaluation. Her past medical history was significant for a stroke in 2003, prior to her diagnosis of PD, which left her with residual left sided weakness. She reported walking on the treadmill at the gym prior to her PD diagnosis, but stated that she always had to use the handrail due to her fear of falling. After her diagnosis, she became more sedentary, participating mainly in seated activities such as tutoring and sewing and only occasionally exercised by walking around her neighborhood. At the time of her initial evaluation with us, she stated her goal was to improve her balance and ability to walk in her neighborhood.
Clinical Impression and Examination
Given the dual diagnosis of PD and a prior stroke, we were concerned that deficits in her gait and balance were contributing to her inability to be as active in the community as she wanted. The prior history of stroke, also caused us to consider the extent of residual hemiparesis as well as the presence of any gait and balance asymmetries. During her initial evaluation she scored a 19/28 on the Mini-BESTest, a 10/14 on the Freezing of Gait Questionnaire, and a 29/30 on the Montreal Cognitive Assessment. She performed the Four Square Step Test in 11.08 seconds and had a total of 30 (15 on Left and 15 on Right) for the Step Test. During the 6MWT (Table 3), we observed that her gait had a decreased stride length on the left side and she ambulated with a wide base of support. Additionally, she ambulated with decreased gait speed, a decreased step length, and decreased arm swing on the left side. We hypothesized that the wide base of support and decreased stride length were due to impairments in balance, while the decreased step length and arm swing on the left were likely due to residual weakness (not tested) from her previous stroke. She performed similarly during her screening and pre-test 6MWT.
Intervention
In an attempt to improve mobility for all three participants, we implemented intensive gait training, with a combination of treadmill-based and overground walking. To address issues with spatiotemporal aspects of gait, we incorporated a novel pairing of rhythmic auditory feedback. We expected that improved spatiotemporal gait parameters would contribute to enhanced balance and balance perception.
Over 6 weeks, participants 1 and 3 completed a total of 15 sessions and participant 2 completed 16 sessions. Each session lasted approximately 60 minutes. During each session, participants began with treadmill-based gait training and ended with overground walking. All of the overground training was performed in a series of flat hallways indoors. We used a metronome during both the overground and treadmill-based components in order to encouraged improvements in both cadence and step length. We set the metronome to a percentage of the participant’s comfortable overground cadence, as determined at the initial evaluation. Importantly, we used different frequencies during treadmill and overground walking. Specifically, when participants walked on the treadmill, we set the metronome to 85% of the comfortable overground cadence, to encourage increased step lengths. Following treadmill walking, we transitioned the participants to overground walking with the metronome set to 115% of the baseline cadence. The overground portion was intended to promote increased gait speed with greater cadence and greater step lengths. The frequency set by the metronome was kept similar throughout the 6 weeks of training despite changes in treadmill speed. Participant 1 completed the treadmill-based training with the metronome set to 89 beats per minute (bpm) and completed overground training with a metronome frequency of 120 bpm. Participant 2 performed treadmill gait training with the metronome set to 115 bpm and completed overground training with the metronome at 153 bpm. For participant 3, the metronome was set to 87 bpm and 117 bpm for treadmill-based training and overground training, respectively.
We set a goal to reach 20 minutes of walking during both overground and treadmill training to maximize the benefits of the intervention; however, all of the participants began with varying times and gradually increased the duration of training. During the initial training session, participants 1 and 2 both completed 15 minutes of walking on the treadmill with one rest break, and were able to complete 20 minutes without a break by the end of the 6 weeks. Participant 3 began by ambulating for a total of 10 minutes with one rest break on the treadmill during the initial session and ended the 6 weeks by ambulating for a total of 20 minutes with one break. The second part of each training session consisted of overground gait training with the metronome. Initially, participant 1 completed 15 minutes of walking overground with one rest break but he progressed to 20 minutes without a break by the last session. Participant 2 walked for 10 minutes overground with one rest break during the initial session but she was able to walk 20 minutes without a break at the final training session. Participant 3 completed 10 minutes of walking with one break at the beginning of the 6 weeks and was able to ambulate a total of 13 minutes without a rest break during the last session.
Blood pressure and heart rate were assessed for each participant at the beginning and end of each session, as well as during each rest break. The intensity of the training was determined by the participants’ physiologic response to exercise (i.e., heart rate), as well as their subjective reports given on the Borg Scale (13-17 on the 6 to 20 scale) measuring their Rate of Perceived Exertion (RPE).25 Over the course of the 6 weeks of training, the treadmill speed was gradually increased in order to allow participants to progress gait speed and step length. Participant 1 began with a gait speed of 1.2 m/s during the initial training session and completed training at a speed of 1.4 m/s during the final session. Participant 2’s treadmill speed began at 1.3 m/s during the first session and was 1.5 m/s at the end of the 6 weeks. Participant 3 began with a treadmill speed of 0.95 m/s but only progressed to a speed of 1.0 m/s by the final session. Although we attempted 1.05 m/s after three weeks of training, we reduced back to 1.0 m/s due to her inability to step with the metronome at that speed. All participants wore a safety harness that was attached overhead to an unweighting system; however, no unweighting was used with any of them throughout the course of training.
While walking, each participant also received verbal feedback throughout each session in regards to his or her spatiotemporal measures of gait. Participants 1 and 2 both received feedback regarding decreased step length bilaterally, whereas participant 3 received feedback more specifically about her decreased step length on the left. Additionally, feedback was provided to participants 1 and 3 in regards to maintaining an upright posture due to their tendency to lean forward during ambulation on the treadmill. Each participant was also provided with verbal feedback in order to achieve heel strike on beat with the metronome.
At the beginning of training, all participants held onto the handrails while walking on the treadmill, although, we discouraged use of the handrails for all participants to challenge balance. Participants 1 and 2 progressed to no handrail use by the end of the 6 weeks; however, participant 3 continued to use both handrails throughout the training program. Although participant 3 attempted to hold onto the therapist’s hand, as a progression towards complete removal of her hand from the rail, she was unable to maintain her balance on the treadmill with this technique. Additionally, when ambulating without upper extremity support, participant 3 reported left shoulder pain due to her tendency to hold her arms in a high guard position.
We experienced a single adverse event that was not an unanticipated problem throughout the 6-week training period. During week 4, participant 3 reported a feeling of lightheadedness after completing the treadmill training and exhibited a drop in blood pressure to 86/62 mmHg. After 10-15 minutes of rest, her blood pressure improved; however, the session was stopped early and no overground training was completed that session. In addition, each participant reported at some point throughout training that they had forgot to take their medication prior to the training session. In these instances, the participants reported the treadmill training was more difficult, however there were no noticeable differences in their performance.
Outcomes
All of the participants repeated all of the gait and balance testing within one week after the last training session, as well as 3 months after training ended. All of the spatiotemporal measures are shown for participant 1 in Table 1, in Table 2 for participant 2, and Table 3 for the third participant.
Participant 1 improved his gait speed from 1.21 m/s at the pretest to 1.48 m/s at the post-test, which was maintained at 1.47 m/s at followup. In addition to improvements in gait speed, participant 1 demonstrated improvements in 6MWT distance, cadence, and stride length. The Minimum Detectable Change (MDC) for patients with PD is identified as 0.18 m/s for gait speed, 82 m for the 6MWT, and 15.1 steps per minute for cadence.26,27 Participant 1 demonstrated an improvement in stride length of 20.5 cm at the post-test compared to the pre-test. At the 3-month follow-up, improvements in all of these measures were still significantly improved compared to the baseline measures taken prior to the study, even with a 4 cm decline in stride length from the post-test measures. Participant 2 ambulated with a comfortable gait speed of 1.31 m/s at baseline and ambulated at a speed of 1.51 m/s at the post-test. During the post-test, she also demonstrated improvements in 6MWT distance, cadence and stride length. At the post-test, her stride length was 137.6 cm compared to 124 cm at the pre-test session. When evaluated during the 3-month follow-up, participant 2 demonstrated improvements in gait speed, cadence, and stride length compared to her post-test measures. Her stride length at the follow-up was 138.2 cm, cadence was 139.6 steps per minute, and her gait speed was 1.55 m/s. Similar to the other two participants, participant 3 demonstrated improvements on the post-test in terms of gait speed, stride length, cadence, and 6MWT distance. Participant 3 ambulated with a gait speed of 0.96 m/s at the baseline testing and walked at 1.14 m/s at post-test. She improved her stride length from 118 cm before training to 131 cm at the post-test. Participant three also improved in her gait speed and stride length at the follow-up, increasing to 1.22 m/s and 140 cm, respectively. She also made slight improvements in her balance measures. The changes seen at follow-up for gait speed and stride length, showed further significant changes from her pretraining results.
Most of the balance measures captured some small improvements at the posttest for all of the participants. Participant 1 demonstrated improvements in balance on the following tests: (Step Test: +13; 4 Square Step Test: -4 s; Freezing of Gait Questionnaire: -3). Participant 2 showed small improvements in balance with these scores (Mini-BESTest: +3; Step Test: +11; 4 Square Step Test: -1.22 s; Freezing of Gait Questionnaire: -1). Participant 3 demonstrated the least improvements in balance with improvements only captured by the Mini-BESTest with an increase in score of 3 points.
At the completion of training, all of the participants reported noticing improvements in their community mobility. Participant 1 reported that instead of ambulating slower than his wife and grandkids, he now often found himself walking slightly ahead of them. Participant 2 reported that she noticed improvements in her stability while walking outside with her husband and in her coordination during dance. She also reported that she noticed she was ambulating with a much quicker pace after the training. Participant 3 reported similar improvements in self-perceived gait speed and stated that she noticed an improved ability to ambulate further distances when walking in her neighborhood.
Table 1.
Participant 1: 6MWT, Gait Speed, and Spatiotemporal and Balance Measures
| Measure | Screening | Pretraining | Posttraining | Follow-up |
| 6MWT distance (m) | 444.7 | 436.17 | 533.1 | 528.8 |
| Gait Speed (m/s) | 1.24 | 1.21 | 1.48 | 1.47 |
| Cadence (steps/minute) | 105 | 114.7 | 114.8 | |
| Stride length (cm) | 144 | 164.5 | 160.2 | |
| Mini-BESTest (max score: 28) | 27 | 27 | 28 | |
| Step Test (reps) | 25 (12 on L 13 on R) | 38 (19 on L and R) | 37 (19 on L 18 on R) | |
| 4 Square Step Test (s) | 11.87 | 7.9 | 7.18 | |
| Freezing of Gait | 7 | 4 | 4 |
Table 2.
Participant 2: 6MWT, Gait Speed, and Spatiotemporal and Balance Measures
| Measure | Screening | Pretraining | Posttraining | Follow-up |
| 6MWT distance (m) | 451.4 | 472.1 | 543.8 | 557.5 |
| Gait Speed (m/s) | 1.25 | 1.31 | 1.51 | 1.55 |
| Cadence (steps/minute) | 132.8 | 136.4 | 139.6 | |
| Stride length (cm) | 124 | 137.6 | 138.2 | |
| Mini-BESTest (max score: 28) | 25 | 28 | 28 | |
| Step Test (reps) | 41 (21 on L 20 on R) | 52 (26 on L and R) | 52 (26 on L and R) | |
| 4 Square Step Test (s) | 6.35 | 5.13 | 4.94 | |
| Freezing of Gait | 3 | 2 | 2 |
Table 3.
Participant 3: 6MWT, Gait Speed, and Spatiotemporal and Balance Measures
| Measure | Screening | Pretraining | Posttraining | Follow-up |
| 6MWT distance (m) | 354.48 | 346.25 | 411.48 | 438.91 |
| Gait Speed (m/s) | 0.98 | 0.96 | 1.14 | 1.22 |
| Cadence (steps/minute) | 102 | 109 | 111 | |
| Stride length (cm) | 118 | 131 | 140 | |
| Mini-BESTest (max score: 28) | 19 | 22 | 23 | |
| Step Test (reps) | 30 (15 on L and R) | 28 (13 on L 15 on R) | 34 (17 on L 17 on R) | |
| 4 Square Step Test (s) | 11.08 | 11.1 | 9.29 | |
| Freezing of Gait | 10 | 10 | 9 |
Discussion
The purpose of this study was to perform an exploratory analysis of the effect of gait training with targeted rhythmic auditory stimulation on gait speed, cadence, stride length, and static and dynamic balance. Ultimately, we found that after participants trained both overground and on the treadmill with targeted rhythmic auditory stimulation, all three participants demonstrated improvements in spatiotemporal gait parameters. Indeed, after 6 weeks of training we observed improvements in gait speed, cadence and stride length compared to baseline. These improvements persisted or further increased at a three month follow up. In addition, some small improvements in balance were also noted for all of the participants at the end of training. This exciting finding has important implications for modifying gait of individuals with Parkinson’s disease through targeted rehabilitation.
The use of rhythmic auditory stimulation during gait training is not a novel intervention, as it has been examined in prior studies both overground and on a treadmill.4,14,28,29 However, the use of different metronome frequencies to specifically target key components of gait had not been evaluated prior to this study. Here, the frequency of the metronome was slower than the participant’s baseline cadence during treadmill training and faster during the overground training. In contrast, previous research has promoted the use of a higher frequency metronome exclusively.3,14 Instead, our use of a slower frequency during treadmill walking, as a part of the training, likely contributed to the large improvements in stride length in our participants.15
There is a lack of current evidence regarding the influence of gait training with rhythmic auditory stimulation on balance outcomes. Although our primary purpose was to ascertain how targeted gait training would affect particular gait parameters, we also sought to determine if there were any improvements in balance following training. Prior work has suggested a carryover of some gait training protocols to improvements in balance.30–35 For example, gait training with cueing resulted in significant improvements in balance as assessed with the Berg Balance Scale (BBS).16,36 The results from our study suggest that there were small improvements in balance for all participants, however the improvements may not be clinically significant.17 The current literature for the psychometric properties of the Four-Square Step test is only available for individuals with Huntington’s disease.37 If applied to our participants, then only participant one successfully improved his time by an amount greater than the MDC. The MDC for the Mini-BESTest is 5.5 points for people with PD, which none of our participants achieved.17 Because of participants 1 and 2’s high initial score on the Mini-BESTest, improving by 5.5 points would not have been possible to achieve. Unfortunately, there is a lack of literature for detectable change in the step test, however participants 1 and 2 both improved by 13 and 11 repetitions respectively, which may be a considerable amount of improvement. It is important to note that the participants were fairly high functioning, so ceiling effects of the selected tests may have masked any potential improvements in balance. Instead, future work should incorporate instrumented analyses of dynamic balance to further assess the potential impact of gait training on balance in individuals with PD.
At the end of training, all three participants demonstrated improvements in their comfortable gait speed (Graph 1). The minimal detectable change (MDC) for gait speed for individuals with Parkinson’s disease is commonly cited as 0.18 m/s.26 At the time of the posttest, all of the participants exceeded this value when compared to their pretest measures with participant 1 exhibiting a change of 0.23 m/s, participant 2 exhibiting a change of 0.30 m/s, and participant 3 exhibiting a change of 0.24 m/s. Furthermore, such large changes in gait speed exceed those reported in previous studies.8,14 Potentially more important, however, was the subjective reports from each participant, as they reported noticing an increase in gait speed when ambulating in the community. As an example, participant 2 reported that her husband no longer walks with her because he complains that she is walking too far ahead of him now. It is likely that the progressive nature of the training, coupled with the targeted approach to enhance both stride length (via low tempo RAS on the treadmill) and cadence (via high tempo RAS overground) contributed to these improvements in gait speed.
Additionally, all participants increased stride length at the end of 6 weeks of training (Graph 2). The MDC for stride length in individuals with Parkinson’s disease is 14 cm.38 Participant 1 exceeded this measure with a change of 20.5 cm; however, participants 2 and 3 were just under the MDC at post-test, but exceeded the MDC by the 3-month follow-up. Nevertheless, the improvements made by all 3 participants exceeded those found in several prior studies that used auditory cueing with this population, at least in terms of the improvements made by the 6 week mark.8,14,39 In fact, our participants improvements were nearly double the stride length improvements reported by others.14 We believe that the biggest contributor to these robust increases in stride length was the slowing of the metronome tempo during the treadmill training. As the treadmill continued to move at the same fast speed, participants took fewer steps in order to match the metronome, thus requiring them to increase their stride length to stay centered on the treadmill. Furthermore, the MDC for cadence in this population is 15.1 steps per minute.27 Despite showing improvements in cadence, none of our participants exceeded that value (Graph 3). These limited improvements in cadence appear to be similar to the findings reported in previous studies; highlighting that while some mild improvements might exist, they may not be clinically significant.8,14
The small sample size of our case series clearly limits the ability to generalize these results to other individuals with Parkinson’s disease. Furthermore, all of the participants had the same stage of disease; therefore, the results cannot be generalized to other H&Y stages. It is also important to note that the training required three, one hour sessions each week for 6 weeks, which may not be feasible in an outpatient setting. Due to the lack of a control group, we cannot determine causation with our study design. It is possible that participants would have improved their gait with training that did not include RAS targeted to key gait parameters. Further research will be needed to ascertain the contributing factors to the observed gait improvements.
In conclusion, this case series describes the novel use of targeted rhythmic auditory stimulation as an intervention to improve gait and balance in individuals with Parkinson’s disease. Ultimately, all three participants demonstrated improvements in gait speed and other spatiotemporal measures (e.g., cadence and stride length) by the end of 6 weeks of training, with these changes persisting for at least 3 months. While slight improvements were noted in the participants’ balance measures, further research is warranted to determine the potential impact that RAS and gait training have on dynamic balance. The outcomes demonstrated by these participants is encouraging for future research on this topic.
Graph 1
Gait Speed for Participants 1, 2, 3 from Pretraining to 3 month Follow Up
Graph 2
Stride Length for Participants 1, 2, 3 from Pretraining to 3 month Follow Up
Graph 3
Cadence for Participants 1, 2, 3 from Pretraining to 3 month Follow Up
References
- Kalia LV, Lang AE. Parkinson’s disease. The Lancet 2015;386(9996):896-912. doi:10.1016/S0140-6736(14)61393-3.
- Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, Brault JM. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord. 1996;11(2):193-200. doi:10.1002/mds.870110213.
- Suteerawattananon M, Morris GS, Etnyre BR, Jankovic J, Protas EJ. Effects of visual and auditory cues on gait in individuals with Parkinson’s disease. J. Neurol. Sci. 2004;219(1-2):63-69. doi:10.1016/j.jns.2003.12.007.
- Harro CC, Shoemaker MJ, Frey OJ, et al. The effects of speed-dependent treadmill training and rhythmic auditory-cued overground walking on gait function and fall risk in individuals with idiopathic Parkinson’s disease: a randomized controlled trial. NeuroRehabilitation 2014;34(3):557-572. doi:10.3233/NRE-141051.
- Sethi K. Levodopa unresponsive symptoms in Parkinson disease. Mov. Disord. 2008;23 Suppl 3:S521-33. doi:10.1002/mds.22049.
- Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease. Mov. Disord. 2015;30(10):1361-1370. doi:10.1002/mds.26269.
- Freedland RL, Festa C, Sealy M, et al. The effects of pulsed auditory stimulation on various gait measurements in persons with Parkinson’s Disease. NeuroRehabilitation 2002;17(1):81-87.
- Rocha PA, Porfírio GM, Ferraz HB, Trevisani VFM. Effects of external cues on gait parameters of Parkinson’s disease patients: a systematic review. Clin Neurol Neurosurg 2014;124:127-134. doi:10.1016/j.clineuro.2014.06.026.
- Ashoori A, Eagleman DM, Jankovic J. Effects of auditory rhythm and music on gait disturbances in parkinson’s disease. Front. Neurol. 2015;6:234. doi:10.3389/fneur.2015.00234.
- Peterson DS, Smulders K. Cues and attention in parkinsonian gait: potential mechanisms and future directions. Front. Neurol. 2015;6:255. doi:10.3389/fneur.2015.00255.
- Gómez-González J, Martín-Casas P, Cano-de-la-Cuerda R. Effects of auditory cues on gait initiation and turning in patients with Parkinson’s disease. Neurologia 2016. doi:10.1016/j.nrl.2016.10.008.
- Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Treadmill walking as an external pacemaker to improve gait rhythm and stability in Parkinson’s disease. Mov. Disord. 2005;20(9):1109-1114. doi:10.1002/mds.20507.
- Thumm PC, Maidan I, Brozgol M, et al. Treadmill walking reduces pre-frontal activation in patients with Parkinson’s disease. Gait Posture 2018;62:384-387. doi:10.1016/j.gaitpost.2018.03.041.
- Thaut MH, Rice RR, Braun Janzen T, Hurt-Thaut CP, McIntosh GC. Rhythmic auditory stimulation for reduction of falls in Parkinson’s disease: a randomized controlled study. Clin. Rehabil. 2019;33(1):34-43. doi:10.1177/0269215518788615.
- Chawla G, Wygand M, Browner N, Lewek MD. Music and Metronome Cueing Across A Range of Frequencies Affects Gait for Individuals with Parkinson’s Disease. J Appl Biomech.
- Harro CC, Shoemaker MJ, Frey O, et al. The effects of speed-dependent treadmill training and rhythmic auditory-cued overground walking on balance function, fall incidence, and quality of life in individuals with idiopathic Parkinson’s disease: a randomized controlled trial. NeuroRehabilitation 2014;34(3):541-556. doi:10.3233/NRE-141048.
- Leddy AL, Crowner BE, Earhart GM. Utility of the Mini-BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J Neurol Phys Ther 2011;35(2):90-97. doi:10.1097/NPT.0b013e31821a620c.
- Giladi N, Tal J, Azulay T, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov. Disord. 2009;24(5):655-661. doi:10.1002/mds.21745.
- Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov. Disord. 2008;23(7):1043-1046. doi:10.1002/mds.22017.
- Duncan RP, Earhart GM. Four square step test performance in people with Parkinson disease. J Neurol Phys Ther 2013;37(1):2-8. doi:10.1097/NPT.0b013e31827f0d7a.
- Mercer VS, Freburger JK, Chang S-H, Purser JL. Step Test scores are related to measures of activity and participation in the first 6 months after stroke. Phys. Ther. 2009;89(10):1061-1071. doi:10.2522/ptj.20080368.
- Godinho C, Domingos J, Cunha G, et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J Neuroeng Rehabil 2016;13:24. doi:10.1186/s12984-016-0136-7.
- Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004.
- Morris M, Iansek R, Matyas T, Summers J. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov. Disord. 1998;13(1):61-69. doi:10.1002/mds.870130115.
- Penko AL, Barkley JE, Koop MM, Alberts JL. Borg scale is valid for ratings of perceived exertion for individuals with Parkinson’s disease. Int J Exerc Sci 2017;10(1):76-86.
- Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys. Ther. 2008;88(6):733-746. doi:10.2522/ptj.20070214.
- Lang JT, Kassan TO, Devaney LL, Colon-Semenza C, Joseph MF. Test-Retest Reliability and Minimal Detectable Change for the 10-Meter Walk Test in Older Adults With Parkinson’s disease. J Geriatr Phys Ther 2016;39(4):165-170. doi:10.1519/JPT.0000000000000068.
- Chaiwanichsiri D, Wango W, Kitisomprayoonkul W, Bhidayasiri R. Treadmill training with music cueing: a new approach for Parkinson’s gait function. Asian Biomedicine 2011;5(5):649-654.
- Ghai S, Ghai I, Schmitz G, Effenberg AO. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci. Rep. 2018;8(1):506. doi:10.1038/s41598-017-16232-5.
- Bryant MS, Workman CD, Hou J-GG, Henson HK, York MK. Acute and Long-Term Effects of Multidirectional Treadmill Training on Gait and Balance in Parkinson Disease. PM R 2016;8(12):1151-1158. doi:10.1016/j.pmrj.2016.05.001.
- Grecco LAC, Tomita SM, Christovão TCL, Pasini H, Sampaio LMM, Oliveira CS. Effect of treadmill gait training on static and functional balance in children with cerebral palsy: a randomized controlled trial. Braz J Phys Ther 2013;17(1):17-23. doi:10.1590/S1413-35552012005000066.
- Tally Z, Boetefuer L, Kauk C, Perez G, Schrand L, Hoder J. The efficacy of treadmill training on balance dysfunction in individuals with chronic stroke: a systematic review. Top Stroke Rehabil 2017;24(7):539-546. doi:10.1080/10749357.2017.1345445.
- Lau KWK, Mak MKY. Speed-dependent treadmill training is effective to improve gait and balance performance in patients with sub-acute stroke. J Rehabil Med 2011;43(8):709-713. doi:10.2340/16501977-0838.
- Yoon SK, Kang SH. Effects of inclined treadmill walking training with rhythmic auditory stimulation on balance and gait in stroke patients. J Phys Ther Sci 2016;28(12):3367-3370. doi:10.1589/jpts.28.3367.
- Mochizuki L, Bigongiari A, Franciulli PM, et al. The effect of gait training and exercise programs on gait and balance in post-stroke patients. Medical Express 2015;2(4). doi:10.5935/MedicalExpress.2015.04.01.
- Song JH, Zhou PY, Cao ZH, Ding ZG, Chen HX, Zhang GB. Rhythmic auditory stimulation with visual stimuli on motor and balance function of patients with Parkinson’s disease. Eur Rev Med Pharmacol Sci 2015;19(11):2001-2007.
- Kloos AD, Fritz NE, Kostyk SK, Young GS, Kegelmeyer DA. Clinimetric properties of the Tinetti Mobility Test, Four Square Step Test, Activities-specific Balance Confidence Scale, and spatiotemporal gait measures in individuals with Huntington’s disease. Gait Posture 2014;40(4):647-651. doi:10.1016/j.gaitpost.2014.07.018.
- Strouwen C, Molenaar EALM, Keus SHJ, Münks L, Bloem BR, Nieuwboer A. Test-Retest Reliability of Dual-Task Outcome Measures in People With Parkinson Disease. Phys. Ther. 2016;96(8):1276-1286. doi:10.2522/ptj.20150244.
- Bella SD, Benoit C-E, Farrugia N, et al. Gait improvement via rhythmic stimulation in Parkinson’s disease is linked to rhythmic skills. Sci. Rep. 2017;7:42005. doi:10.1038/srep42005.
Evaluation
While writing our manuscript we received feedback from Dr. Lewek as we completed each section and incorporated that feedback until we made our final product. Afterwards, we sent our manuscript to our committee who emailed us back with feedback. The posted manuscript incorporates feedback from Diane Meyer, which was about occasional wording choices and adding in reference values for some of the outcome measures included in the study. We also received feedback from the UNC PT faculty and students who came to our poster presentation at the Human Movement Science Research Symposium. We gave them an evaluation form which they filled out after viewing our poster. The feedback from our poster presentation was very positive with a very high score for all components.
We are planning on having our manuscript published, so we will also receive feedback in the form of peer-reviews after it is sent in for publication.
Self-Reflection
I never thought I would participate in research and I never even considered that I would write a manuscript that would one day be published, but I am proud to say that I have accomplished just that. I have learned so much through this experience and have a better understanding of how research works and the many components involved with it. I also believe that my skills have grown with analyzing gait and its spatiotemporal measures and seeing firsthand how physical therapy can help to change those aspects in patients with Parkinson’s disease. Seeing the progress that the patients make and hearing how they feel the interventions are working in their lives really makes the research rewarding, not to mention all of the future patients that will benefit from it. Even though I plan to work in a clinic after graduation, I am open to research in the future after this wonderful experience!
Image source: https://www.fleetfeet.com/blog/treadmill-workouts


3 Responses to “Rhythmic Auditory Stimulation for Improved Gait in Parkinson’s Disease”
Sarah Stevenson
Debbie- Thank you for your kind words! I really enjoyed working on this project and we are planning on submitting our manuscript for publication this summer. Writing the manuscript was a feat I never thought I would accomplish, so we will see what the future holds for my involvement with research.
Lewek- Thank you so much for all of your help throughout this past year with conducting our research and giving us feedback on our manuscript. Without your guidance this project would not have gone as smoothly as it did. Your suggestion of breaking the manuscript into sections and working on small parts helped to make the task of writing the whole manuscript less intimidating. The results that we found were very encouraging and I hope that you are able to expand this research in the future!
Michael Lewek
Sarah
I’m very proud of the work that you and Ali did together. You pulled together an amazing manuscript that we will submit to JNPT this summer. You both did an excellent job in synthesizing the literature and interpreting your findings in the broader context of the available clinical literature. The feedback that you received so far on your presentation and manuscript has been extremely positive.
I know the idea of writing a publishable manuscript seemed daunting at the beginning of the semester, but you set timeframes for each component and worked diligently to complete each section in a timely manner.
Outstanding work!
BTW, I’m copying the same comment to Ali, considering how closely you have both worked over the past year.
Mike
Debbie Thorpe
Sarah
You and Ali did a great job on this manuscript and poster for HMSC Day! I hope you are going to submit the manuscript for publication. I think you both have more research in your future! Very informative, well organized, professional!