The Role of Verbal Instruction in Immediately Altering Spatiotemporal
Gait Parameters Post-Stroke
By: Hailey Guerin, SPT
BACKGROUND
Throughout all of my clinical rotations thus far during physical therapy school, I have repeatedly encountered patients who were recovering from a stroke. Whether it was immediately following the stroke as seen in the hospital, a few weeks later at inpatient rehabilitation or several months later in an outpatient clinic, I was never lacking exposure to treating patients who were recovering from this life-changing condition. As a future physical therapist, one of the most important skills that we help patients with, particularly those who have sustained a stroke, is the ability to walk. While gait symmetry, weight-shifting, balance and appropriate use of leg musculature are extremely important to us, one of the most important gait characteristics as perceived by the patients is the ability to quickly walk. Thus, in order to make efficient use of everyone’s time during therapy, both for therapists and the patients, I have chosen to complete research in determining which types of feedback during gait training produce the best outcomes in spatiotemporal gait characteristics. Hopefully, our research will help future and current clinicians better determine how to cue and train their patients recovering from stroke in order to produce the desired outcomes necessary to appease our patients’ concerns during their recovery process.
ACKNOWLEDGEMENTS
I cannot express enough thanks to my committee team for their continued support and encouragement. My completion of this project could not have been possible without the help of Dr. Michael Lewek, PT, PhD whose passion and diligence in the medical field not only motivated myself to become a better researcher but also as a future clinician. Additionally, my fellow colleague, research partner and best friend, Ben Buchanan, SPT, made this work possible by consistently demonstrating a tireless work ethic as well as an optimistic and uplifting attitude throughout this year of research. Furthermore, I would also like to thank Dr. Vicki Mercer, PT, PhD and Dr. Katie Marakas PT, DPT, CLT, CSRS for taking the time out of their busy schedules to provide valuable feedback and support along the way. It is an absolute honor to have learned from all of the faculty members at UNC’s DPT program, whose knowledge and genuine compassion for helping others served as a constant reminder of why I chose to pursue this field of study.
ABSTRACT
Introduction: Individuals with hemiparesis post-stroke commonly demonstrate gait abnormalities that lead to difficulty with community ambulation and an increased risk of falls. Physical therapy plays an important role in improving gait after stroke, often including the use of verbal feedback; however, the immediate effect of verbal feedback has not yet been quantified. Because evidence suggests that tactile, verbal and visually cued feedback are able to improve step length asymmetry and gait speed with repeated sessions of gait-training, we believe that we can use specific verbal cueing to alter spatiotemporal parameters for individual’s paretic limbs as well as increase gait speed.
Objective: The purpose of this study was to determine the immediate effect of verbal commands during gait training and to see if individuals post-stroke can take longer step lengths, greater cadences, increased paretic stance times, and faster speeds than ‘typical’ walking when given specific commands.
Methods: Twenty-nine participants with chronic stroke (>6 months post-stroke) participated in this study. Each participant completed a single-session evaluation which consisted of taking a baseline measurement of their “normal” walking, followed by walking with six different verbal cues to assess the effect of the cues on gait parameters. Participants were allowed to use their normal assistive devices and ankle-foot-orthoses as they would use at home or within the community setting. Patients were guarded with stand-by assistance for any minor losses of balance to prevent falls. Data was collected using a GAITRite mat to assess various gait parameters including: step length, cadence and gait speed.
Results: Individuals following stroke showed significant increases in step lengths, gait speed, cadences and paretic stance times when given appropriate and specific verbal cues. While some cues were able to increase the participants’ cadences and paretic stance times, other cues were able to elicit the opposite effect. There was no significant effect of verbal instruction on step length asymmetry or stance time asymmetry.
Conclusion: Individuals with chronic hemiparesis following stroke have the capacity to alter their gait performance to specific verbal instruction in a single session even amongst survivors with a wide range of stroke recovery. Participants were immediately able to elicit increases in step length, cadences, paretic stance times and faster speeds when given specific cues without using prolonged interventions or training programs. Clinicians may immediately modify a patient’s gait by using specific verbal cues to achieve desired outcomes based on treatment goals.
MANUSCRIPT (PDF)
Background
Strokes are a leading cause of long-term disability in the US with up to 66% of affected individuals having disability several years following a stroke.1,2 Residual impairments seen in stroke survivors that may lead to reduced levels of activity and further disability include: hemiparesis, spasticity and cognitive dysfunction.3 Stroke-related damage within the brain can potentially influence visuospatial and motor attention thus affecting motor performance contributing to a variety of balance and gait deficits. In addition to having delayed and less coordinated responses to self-induced and external balance perturbations, individuals may also experience a reduced ability of propulsion at push-off, decreased hip flexion, knee flexion, gait speed, step length and altered cadences and stance/swing times during the gait cycle.4,5,6
Abnormalities within spatiotemporal measures may lead to an increased risk of falls in individuals post-stroke.6 Among patients with stroke, the fall frequency rates among general hospitals and nursing homes have been reported to be 25 to 39%, with advanced age contributing as an additional risk factor.7 With approximately 795,000 new cases of strokes occurring each year coupled with the fact that the risk of having a stroke doubles each decade after the age of 55, it is important for physical therapists and other healthcare providers to utilize effective treatment strategies for addressing patients’ specific deficits during their recovery from stroke.1
Although it has been documented that cuing is common practice in gait rehabilitation, there is not much in the literature about the specific effects of verbal cuing on gait rehabilitation post-stroke or what specific cuing is most effective.8,9 Spatiotemporal gait parameters are modifiable with training.10 Clearly gait is modifiable, as evidenced by a robust walking speed reserve, giving individuals the ability to walk faster than they typically do.11 Additionally, Reisman et. al found that individuals post-stroke who sustained cerebral damage were able to make motor adaptations regardless of the severity of sensorimotor deficits.12 This is important as it suggests that individuals’ style of walking is not due to fixed neural circuitry, as individuals were able to alter movements towards “normal” walking. Clinically, we can use verbal cuing to change gait parameters within individuals post-stroke. A recent clinical trial attributed altered spatiotemporal gait parameters to verbal cuing; however, the effect of various cues on specific parameters remains unknown.10 Additionally, the ability of individuals post-stroke to make immediate changes to spatiotemporal parameters from simple verbal commands remains unknown.10,13,14 Given the need to know the effect of verbal commands on gait to best improve gait training, our study investigated specific verbal cues during over-ground walking.
Previous studies have shown that patients more than six months post-stroke who are able to walk were able to improve step length asymmetry following training with tactile, verbal and visually cued feedback with repeated sessions of gait-training but not through one session alone.10,15 Ploughman et. al found that verbal cues were better able to consistently increase the EMG activity of the vastus lateralis and medial gastrocnemius muscles involved with propulsion during the gait cycle, and were able to produce statistically and clinically significant improvements in the participants’ gait speed.16 Unfortunately, those authors didn’t use consistent cues across subjects, leaving them unable to determine the effect of specific verbal cues on the ability to change gait. Nevertheless, this finding suggests that therapists are able to influence muscle activation patterns in individuals in ways that may not be achieved through individual practice alone.
The multiple gait patterns observed following a stroke will likely require multiple approaches. Given that individuals are capable of producing changes in their gait post-stroke, determining the specific effects of verbal commands on gait will allow therapists to tailor their treatments to address patient’s specific deficits.
The purpose of this study was to determine the immediate effect of verbal commands on spatiotemporal gait parameters of both the paretic and non-paretic limbs. We hypothesized that individuals post-stroke would have the capacity to alter their gait to specific verbal instruction intended to elicit longer step lengths, greater cadences, increased paretic stance times, and faster speeds than ‘typical’ walking. The outcomes of this study are expected to help guide clinical practice in the physical therapy setting by providing evidence for the role of verbal cues in altering gait for individuals following stroke.
Methods
Participants
Individuals recovering from a stroke were recruited for participation in this study from various stroke support groups across North Carolina. Participants were excluded if their stroke occurred within 6 months of testing, if the stroke affected the brainstem or cerebellum, or if the patient demonstrated significant receptive aphasia. Additionally, participants were excluded due to having other neurologic or orthopedic disorders that might affect the ability to walk, a history of balance deficits or unexplained falls not related to the stroke, or uncontrolled seizures. Participants had to be able to ambulate 20 feet with at least standby-assist (SBA). All participants signed an informed consent form approved by the IRB of the University of North Carolina at Chapel Hill before participating.
Protocol
All testing occurred in a single session where participants were instructed to “walk at a comfortable pace” (comfortable gait speed [CGS]) for two passes along a 14’ GAITRite mat (CIR Systems, Franklin, NJ). This initial component was used to establish a baseline for the individuals’ gait parameters. The two passes consisted of walking down the mat one way and back the opposite direction. Participants then repeated walks across the mat for six conditions in random order. The six conditions consisted of different verbal instructions:
(1) “Walk as fast as you can safely”; intended to determine how participants changed their overall speed
(2) “Walk at a comfortable speed while swinging your arms as much as you can”; intended to generate longer steps given the propriospinal connections between arms and legs
(3) “Walk at a comfortable speed while taking as high steps as you can”; intended to encourage weight shift to the stance leg for greater stance times
(4) “Walk at a comfortable speed while taking as long steps as you can”; intended to encourage longer steps
(5) “Push off of the ground as hard as you can with each step”; intended to produce greater step length
(6) “Take as quick of steps as you can”; intended to determine how participants increased their cadence
The eighth, and final command was to again “walk down and back at a comfortable pace”. Participants took rest breaks between conditions, as needed. Participants were allowed to use assistive devices or orthoses during ambulation, if necessary.
If patients were unable to understand the initial command, it was repeated, but no further explanation or demonstration was given to clarify the instruction. Patients were told before testing that we would only give one command per condition and interpretation of the command was dependent on their own judgment.
Data Processing & Statistical Analysis
We used GAITRite software version 4.7 in order to process collected data, which removed any partial steps, toe-drags and assistive devices from each pass across the mat. The software grouped each pass across the mat by the verbal instruction that was provided. For each subject, the following outcome measures were exported: gait speed, cadence, stance time and step length.
Asymmetry ratios were calculated as:
step length asymmetry=max(paretic, nonparetic)/(paretic + non-paretic)
stance time asymmetry=paretic/(paretic+non-paretic)
Outcome measures were analyzed using SPSS version 24. Each outcome measure was compared between conditions using a one-way repeated measures ANOVA, repeated for verbal instruction. When significant main effects were found, we performed paired samples t-tests between the CGS condition and each of the verbal instructions to determine the significance. P-values of less than 0.05 were considered significant.
Results
We recruited twenty-nine participants (12 male; 17 female; mean age 64 +/- 13; mean height of 67” +/- 4”; mean weight 182lbs +/- 40lbs.) who were ≥6 months post-stroke with paretic impairments on one side of their body. Of the participants, fourteen were paretic on their right side and fifteen were paretic on their left side. Thirteen of the participants used an ankle-foot-orthosis (AFO) and/or an assistive device such as a single point cane, quad cane or rolling walker.
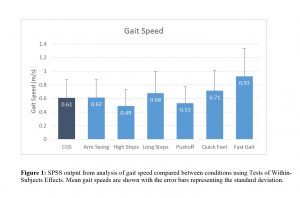
Gait speed was significantly different between conditions (p<0.001; ηp2=0.558; see figure 1). Specifically, compared to the CGS condition, we observed that gait speed was significantly slower when asked to walk with high knees (p=0.014), and significantly faster when asked to walk with quick feet (p=0.011) and when asked to walk as fast as possible (p<0.001).
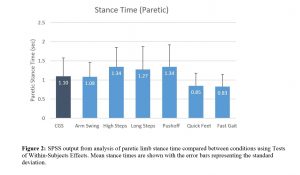
Stance time on the paretic limb was also significantly different between conditions (p<0.001; ηp2=0.507; see figure 2). Participants were able to spend an increased amount of time on their paretic lower limb when asked to walk with high steps, long steps and a greater push-off (all p<0.026). Additionally, asking participants to walk with quick steps and fast walking produced a reduction in the amount of time spent on the paretic limb (both p<0.001).
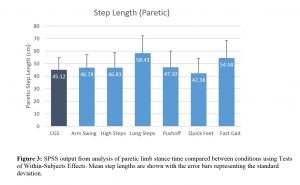
A significant main effect for step length was observed between conditions (p<0.001; ηp2=0.411; see Figure 3) when analyzing the shorter step, regardless of whether it was the paretic or non-paretic limb. Specifically, walking with instruction to take longer steps or to walk as quickly as possible both resulted in participants producing longer step lengths (both p<0.001).
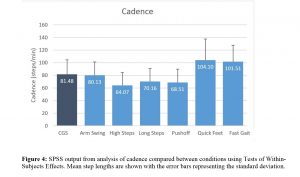
Cadence was found to be significantly different between conditions (p<0.001; ηp2=0.621; see Figure 4). Instructions to produce high steps, long steps and greater push-off significantly reduced the cadence of the participants’ walking (all p<0.005) whereas instructions to walk with quick steps or fast walking significantly increased the participants’ cadence (both p<0.001).
We observed no significant effect of verbal instruction on step length asymmetry (p=.441; ηp2=0.035) or stance time asymmetry (p=.496; ηp2=0.030).
Discussion
Our hypothesis that individuals post-stroke would have the capacity to alter their gait to specific verbal instruction to elicit increases in step length, cadences, paretic stance times and faster speeds was supported. Specifically, we observed that participants were able to alter gait speed, cadence, step lengths and stance times but not spatiotemporal asymmetry. We further noted that these changes were specific to individual verbal commands; each verbal instruction did not alter every gait parameter, indicating the need for specificity of instruction to achieve a desired outcome. When specifically analyzing changes in gait speed, we found that the verbal instructions to walk with quick feet or when asked to walk as fast as possible were able to elicit increases in speed. When given verbal instruction to perform high knees while walking, patients’ walking speeds significantly decreased. While previous studies were able to demonstrate statistically and clinically significant improvements in participants’ gait speeds with verbal cues,16 our study is the first to use consistent cuing methods across all subjects with the same effect.
Importantly, our participants were able to immediately modify their gait based on specific cues, without using specific interventions such as repetitive task practice or a strengthening program, a concept that has not yet been established in previous studies. As an example, motor adaptation paradigms allow individuals following stroke to induce changes within spatial or temporal parameters to drive optimal walking patterns.17 While it is known that repeated adaptation can lead to learning a new motor pattern,17 our study was able to demonstrate that individuals are capable of producing altered motor responses in a single, non-repeated session. With as many as 11% of individuals requiring assistance to walk even after completing a rehabilitation program, there is a clear need for methods to elicit changes in walking.18 In the clinic, physical therapists often provide demonstration or correction of movement patterns with other methods such as tactile or visual cueing if the patient is unable to produce the desired outcome or is struggling to comprehend the verbal instructions.19,20,21 Our study supports the efficacy of these instructions and emphasizes the importance of using specific language with cuing as each instruction is capable of influencing specific motor outputs for patients.
Following a stroke, the majority of individuals present with stance and swing time asymmetries due to reductions of the non-paretic limb swing time, a prolongation of the paretic limb swing time or a combination of both.18 Our findings are important as it suggests that clinicians may immediately modify a patient’s gait by using specific verbal cues to achieve desired outcomes based on treatment goals, such as increasing or decreasing the amount of time spent on the paretic limb. Asymmetries in spatial and temporal parameters are commonly present in stroke survivors, indicating that individuals post-stroke develop compensatory mechanisms during locomotion to compensate for paretic muscles and yield “normal” walking patterns.18 During rehabilitation, therapists and healthcare providers may utilize the specific verbal cues within our study to elicit increases or decreases in paretic limb stance times depending on the desired outcome. However, our participants demonstrated a symmetrical adjustment in stance times on both the paretic and non-paretic limbs because our instructions were targeted at changing both lower extremities rather than focusing on unilateral changes. Others have demonstrated the ability of instructions targeting unilateral change to successfully influence spatial asymmetry.22 When participants were asked to produce high steps, long steps or greater push-off while walking, they were able to reduce the cadence of their walking whereas instruction to walk with quick steps or fast walking produced the opposite effect. Clinically, this is important as it suggests that individuals post-stroke are capable of altering their walking rhythm when provided with an appropriate cue.
In conclusion, we observed that individuals post-stroke have the ability to modify their gait parameters when walking, even amongst survivors with a wide range of stroke recovery. Notably, the measures of change that were related to their gait performance involved specific cues for achieving specific outcomes. Additionally, these induced spatiotemporal changes occurred immediately without using specific interventions or training programs.
There are several possible limitations of this study. Given that we decided to investigate how participants naturally comprehended specific verbal cues for given tasks without providing further explanation, it is possible that participants misinterpreted how to perform a desired outcome. In addition to having a wide range of chronicity of strokes within our participants, our study was a single-session design making it impossible to determine the carry-over of effects with long-term changes. In theory, if participants are able to change gait characteristics immediately, they should ideally be able to make long-term changes with repetitive practice as well. Future studies with prolonged sessions and a narrower range of functional recovery within participants are warranted to better evaluate the long-term effects of specific verbal instructions on specific gait outcomes. This study will guide future work in gait training interventions for establishing its viability long-term for post-stroke rehabilitation.
References
- Stroke Statistics | Internet Stroke Center. Strokecenterorg. 2018. Available at: http://www.strokecenter.org/patients/about-stroke/stroke-statistics/.
- Patel M, Tilling K, Lawrence E, Rudd A, Wolfe C, McKevitt C. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing. 2006;35(3):273-279. doi:10.1093/ageing/afj074.
- Billinger S, Arena R, Bernhardt J et al. Physical Activity and Exercise Recommendations for Stroke Survivors: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532-2553. doi:10.1161/str.0000000000000022.
- Weerdesteyn V, Niet M, van Duijnhoven H, Geurts A. The Journal of Rehabilitation Research and Development. 2008;45(8):1195. doi:10.1682/jrrd.2007.09.0145.
- Peters S, Handy T, Lakhani B, Boyd L, Garland S. Motor and Visuospatial Attention and Motor Planning After Stroke: Considerations for the Rehabilitation of Standing Balance and Gait. Phys Ther. 2015;95(10):1423-1432. doi:10.2522/ptj.20140492.
- Lewek M, Bradley C, Wutzke C, Zinder S. The Relationship between Spatiotemporal Gait Asymmetry and Balance in Individuals with Chronic Stroke. Journal of Applied Biomechanics. 2014;30:31-36. doi:10.1123/jab.2012-0208.
- Teasell R, McRae M, Foley N, Bhardwaj A. The incidence and consequences of falls in stroke patients during inpatient rehabilitation: Factors associated with high risk. Arch Phys Med Rehabil. 2002;83(3):329-333. doi:10.1053/apmr.2002.29623.
- van Vliet P, Wulf G. Extrinsic feedback for motor learning after stroke: What is the evidence?. Disability and Rehabilitation. 2006;28(13-14):831-840. doi:10.1080/09638280500534937.
- Nascimento L, de Oliveira C, Ada L, Michaelsen S, Teixeira-Salmela L. Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: a systematic review. J Physiother. 2015;61(1):10-15. doi:10.1016/j.jphys.2014.11.015.
- Lewek M, Braun C, Wutzke C, Giuliani C. The role of movement errors in modifying spatiotemporal gait asymmetry post stroke: a randomized controlled trial. Clin Rehabil. 2018;32(2):161-172. doi:10.1177/0269215517723056.
- Middleton A, Braun C, Lewek M, Fritz S. Balance impairment limits ability to increase walking speed in individuals with chronic stroke. Disabil Rehabil. 2016;39(5):497-502. doi:10.3109/09638288.2016.1152603.
- Reisman D, Wityk R, Silver K, Bastian A. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130(7):1861-1872. doi:10.1093/brain/awm035.
- Stanton R, Ada L, Dean C, Preston E. Feedback Received While Practicing Everyday Activities During Rehabilitation After Stroke: An Observational Study. Physiotherapy Research International. 2014;20(3):166-173. doi:10.1002/pri.1612.
- Johnson L, Burridge J, Demain S. Internal and External Focus of Attention During Gait Re-Education: An Observational Study of Physical Therapist Practice in Stroke Rehabilitation. Phys Ther. 2013;93(7):957-966. doi:10.2522/ptj.20120300.
- Wright R, Brownless S, Pratt D, Sackley C, Wing A. Stepping to the Beat: Feasibility and Potential Efficacy of a Home-Based Auditory-Cued Step Training Program in Chronic Stroke. Frontiers in Neurology. 2017;8. doi:10.3389/fneur.2017.00412.
- Ploughman M, Shears J, Quinton S et al. Therapists’ cues influence lower limb muscle activation and kinematics during gait training in subacute stroke. Disability and Rehabilitation. 2017:1-8. doi:10.1080/09638288.2017.1380720.
- Bastian, A. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21(6):628-633. doi:10.1097/wco.0b013e328315a293.
- Lauzière S, Betschart M, Aissaoui R, Nadeau S. Understanding Spatial and Temporal Gait Asymmetries in Individuals Post Stroke. Int J Phys Med Rehabil. 2014;02(03). doi:10.4172/2329-9096.1000201.
- Keus S, Bloem B, Hendriks E, Bredero-Cohen A, Munneke M. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Movement Disorders. 2007;22(4):451-460. doi:10.1002/mds.21244.
- Lim I, van Wegen E, Jones D, Rochester L, Nieuwboer A, Marie Willems A, Baker K, Hetherington V, Kwakkel G. Does cueing training improve physical activity in patients with Parkinson’s disease? Neurorehabilitation and Neural Repair. 2010;24(5):469-477. doi: 1177/1545968309356294
- Walker C, Brouwer B, Culham E. Use of Visual Feedback in Retraining Balance Following Acute Stroke. Physical Therapy. 2000;80(9):886-895. doi:10.1093/ptj/80.9.886
- Clark DJ, Neptune RR, Behrman AL, Kautz SA. Locomotor Adaptability Task Promotes Intense and Task-Appropriate Output From the Paretic Leg During Walking. Arch Phys Med Rehabil. 2016;97(3):493-496. Doi: 10.1016/j.apmr.2015.10.081.
Image Credit: Brian Pisor

4 Responses to “The Role of Verbal Instruction in Immediately Altering Spatiotemporal Gait Parameters Post-Stroke”
Michael Lewek
Hailey
I’m glad this all came together for you. To answer Vicki’s question, you can look at the SPSS output and look at the asymmetry values for the CGS condition. I can’t remember how we ended up calculating it, but either a value of 1.0 or 0.5 would indicate perfect symmetry (depending on the way I calculated it). Any deviation from that would indicate asymmetry. Values may appear to look close to those values and still be asymmetric. For example if their CGS asymmetry for step length was greater than 0.535, then they would be greater than 2 standard deviations off of ‘unimpaired control subjects’.
Hope that helps.
Hailey
Hi Cheyenne, thank you, it definitely was an interesting project to be involved in! One of the verbal cues provided (“push off of the ground as hard as you can with each step”) seemed to confuse people but we were only permitted to repeat the cue with no additional explanation. For someone who talks with their hands and body, it was very hard to restrain myself from demonstrating what we wanted to produce with the cue!
Hi Dr. Mercer, I think you’re correct in stating that our participants were not asymmetrical to begin with; however, we did not specifically test for this at baseline or throughout the data analysis. I think that would be interesting to analyze in further detail!
Vicki Mercer
Hi Hailey-
Good work on this project! I was wondering about how much asymmetry of gait your participants demonstrated. Could part of the reason that you didn’t see effects on asymmetry be that they were not very asymmetrical to begin with?
Vicki
Cheyenne Brown
Hi Hailey! This looks like it was a very interesting study to be a part of! Like you said, we will see patients who have had a stroke across multiple settings, so having studies that support aspects of our care can help maximize the benefits for patients. I really appreciated the inclusion of the verbal cues that were given to the participants and the intention behind them, that helps me think about the cues I often use and how I can be more deliberate in my wording to meet a specific objective. Were there any noticeable instances where a participant did not appear to understand these cues? I saw that those with significant receptive aphasia were excluded, but I am just thinking of those with perhaps milder changes in cognition and communication/language abilities. You and Ben obviously put a lot of time into this, and it paid off since I think y’all did a great job!